Deck 14: Energy: What Makes Reactions Go
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/22
Play
Full screen (f)
Deck 14: Energy: What Makes Reactions Go
1
Which of the following statements about energy are true? Select all that apply.
A) Energy can be transformed from one form to another.
B) Energy is measured in kelvin, K.
C) Energy is the capacity of an entity to do work.
D) Energy can be destroyed.
E) Energy cannot be created.
A) Energy can be transformed from one form to another.
B) Energy is measured in kelvin, K.
C) Energy is the capacity of an entity to do work.
D) Energy can be destroyed.
E) Energy cannot be created.
A,C,E
2
The kinetic energy of a 1000 kg truck traveling at 20 m s-1 is equal to which of the following?
A) 400 000 J
B) 200 J
C) 2000 J
D) 10 000 J
E) 200 000 J
A) 400 000 J
B) 200 J
C) 2000 J
D) 10 000 J
E) 200 000 J
E
3
How much work needs to be done to lift a 3 kg weight to a height of 3 m?
A) 1 kJ
B) 9 kJ
C) 900 J
D) 90 N
E) 90 J
A) 1 kJ
B) 9 kJ
C) 900 J
D) 90 N
E) 90 J
E
4
What is the enthalpy change for the following reaction (the reaction of ethane with hydrogen bromide to form bromoethane):
CH2CH2 + HBr CH3CH2Br
Use the following bond energies where appropriate:
C-H = 412 kJ mol-1; C-C = 348 kJ mol-1; C=C = 610 kJ mol-1; H-Br = 365 kJ mol-1; C-Br = 280 kJ mol-1.
A) 65 kJ
B) -65 kJ mol-1
C) 5311 kJ mol-1
D) 65 kJ mol-1
E) -327 kJ mol-1
CH2CH2 + HBr CH3CH2Br
Use the following bond energies where appropriate:
C-H = 412 kJ mol-1; C-C = 348 kJ mol-1; C=C = 610 kJ mol-1; H-Br = 365 kJ mol-1; C-Br = 280 kJ mol-1.
A) 65 kJ
B) -65 kJ mol-1
C) 5311 kJ mol-1
D) 65 kJ mol-1
E) -327 kJ mol-1

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
5
If the products of a reaction have a higher Gibbs free energy than the reactants, which of the following statements are true? Select all that apply.
A) The products are more stable than the reactants.
B) The products are less stable than the reactants.
C) The reaction has consumed more energy than it released.
D) The reaction is endergonic.
E) G for the reaction is negative.
A) The products are more stable than the reactants.
B) The products are less stable than the reactants.
C) The reaction has consumed more energy than it released.
D) The reaction is endergonic.
E) G for the reaction is negative.

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements about the enthalpy change of reaction are true? Select all that apply.
A) It has units of J.
B) It is represented by the symbol G.
C) It measures the exchange of energy with the surroundings during the course of the reaction.
D) It is a measure of the bond-breaking and bond-making which occurs during the course of a reaction.
E) It always has a positive value.
A) It has units of J.
B) It is represented by the symbol G.
C) It measures the exchange of energy with the surroundings during the course of the reaction.
D) It is a measure of the bond-breaking and bond-making which occurs during the course of a reaction.
E) It always has a positive value.

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
7
Match the characteristic of a chemical reaction with the name given to that type of reaction.
-ΔH is positive
A) Endothermic
B) Exergonic
C) Endergonic
D) Exothermic
-ΔH is positive
A) Endothermic
B) Exergonic
C) Endergonic
D) Exothermic

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
8
Match the characteristic of a chemical reaction with the name given to that type of reaction.
-ΔG is negative
A) Endothermic
B) Exergonic
C) Endergonic
D) Exothermic
-ΔG is negative
A) Endothermic
B) Exergonic
C) Endergonic
D) Exothermic

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
9
Match the characteristic of a chemical reaction with the name given to that type of reaction.
-ΔG is positive
A) Endothermic
B) Exergonic
C) Endergonic
D) Exothermic
-ΔG is positive
A) Endothermic
B) Exergonic
C) Endergonic
D) Exothermic

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
10
Match the characteristic of a chemical reaction with the name given to that type of reaction.
-ΔH is negative
A) Endothermic
B) Exergonic
C) Endergonic
D) Exothermic
-ΔH is negative
A) Endothermic
B) Exergonic
C) Endergonic
D) Exothermic

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements about heat and energy are true? Select all that apply.
A) An object with a high temperature has more energy than an object with a low temperature.
B) Heat is the transfer of energy from hot to cold.
C) 'Heat' and 'temperature' mean the same thing.
D) The transfer of heat from hot to cold is a non-spontaneous process.
A) An object with a high temperature has more energy than an object with a low temperature.
B) Heat is the transfer of energy from hot to cold.
C) 'Heat' and 'temperature' mean the same thing.
D) The transfer of heat from hot to cold is a non-spontaneous process.

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
12
A mug of tea is an example of which one of the following?
A) An open system.
B) A closed system.
C) An isolated system.
A) An open system.
B) A closed system.
C) An isolated system.

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
13
Which one of the following phrases correctly completes the sentence "Chemical reactions occur spontaneously…"
A) …if H for the reaction is < 0.
B) …if S for the reaction is < 0.
C) …if the Gibbs free energy change for the reaction is positive.
D) …if the Gibbs free energy for the reaction is negative.
E) None of the phrases given
A) …if H for the reaction is < 0.
B) …if S for the reaction is < 0.
C) …if the Gibbs free energy change for the reaction is positive.
D) …if the Gibbs free energy for the reaction is negative.
E) None of the phrases given

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
14
Which one of the following is the correct description of a closed system?
A) Neither matter nor energy can be transferred between the system and surroundings.
B) Only matter can be transferred between the system and surroundings.
C) Only energy can be transferred between the system and surroundings.
D) Both energy and matter can be transferred between the system and surroundings.
A) Neither matter nor energy can be transferred between the system and surroundings.
B) Only matter can be transferred between the system and surroundings.
C) Only energy can be transferred between the system and surroundings.
D) Both energy and matter can be transferred between the system and surroundings.

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following statements about energy are true? Select all that apply.
A) Bond cleavage generates energy.
B) Endothermic reactions absorb energy.
C) Exergonic reactions release energy.
D) Energy can only flow from system to surroundings.
A) Bond cleavage generates energy.
B) Endothermic reactions absorb energy.
C) Exergonic reactions release energy.
D) Energy can only flow from system to surroundings.

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following are the correct units for values of entropy?
A) mol
B) J mol-1
C) mol K-1
D) mol J-1 K-1
E) J K-1 mol-1
A) mol
B) J mol-1
C) mol K-1
D) mol J-1 K-1
E) J K-1 mol-1

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
17
Which one of the following statements about Gibbs free energy is true?
A) All the energy generated by a reaction is free to do work.
B) The Gibbs free energy change for a reaction is not dependent on temperature.
C) If the products of a reaction have less Gibbs free energy than the reactants, the reaction is not spontaneous.
D) A reaction with a negative ΔG cannot happen.
E) Catabolic reactions typically have a negative ΔG.
A) All the energy generated by a reaction is free to do work.
B) The Gibbs free energy change for a reaction is not dependent on temperature.
C) If the products of a reaction have less Gibbs free energy than the reactants, the reaction is not spontaneous.
D) A reaction with a negative ΔG cannot happen.
E) Catabolic reactions typically have a negative ΔG.

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
18
Which one of the following statements does not apply to an endothermic reaction?
A) The enthalpy of the system increases.
B) The entropy of the system increases.
C) Energy flows from the surroundings to the system.
D) The enthalpy of the surroundings decreases.
E) The energy of the universe decreases.
A) The enthalpy of the system increases.
B) The entropy of the system increases.
C) Energy flows from the surroundings to the system.
D) The enthalpy of the surroundings decreases.
E) The energy of the universe decreases.

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
19
Which one of the following is not associated with an increase in entropy?
A) The folding of a polypeptide chain into a more compact three-dimensional shape.
B) The melting of ice to yield liquid water.
C) The evaporation of sweat from the surface of the skin
D) The breakdown of glycogen into its component glucose subunits.
A) The folding of a polypeptide chain into a more compact three-dimensional shape.
B) The melting of ice to yield liquid water.
C) The evaporation of sweat from the surface of the skin
D) The breakdown of glycogen into its component glucose subunits.

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
20
Match the correct words with the numbered gaps in the following relationship:
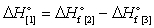
-Products
A) 2
B) 1
C) 3
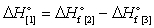
-Products
A) 2
B) 1
C) 3

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
21
Match the correct words with the numbered gaps in the following relationship:
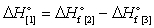
-Reaction
A) 3
B) 2
C) 4
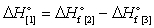
-Reaction
A) 3
B) 2
C) 4

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck
22
Match the correct words with the numbered gaps in the following relationship:
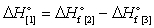
-Reactants
A) 4
B) 3
C) 5
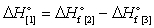
-Reactants
A) 4
B) 3
C) 5

Unlock Deck
Unlock for access to all 22 flashcards in this deck.
Unlock Deck
k this deck


