Deck 12: Chemical Reactions, Oxidation, and Reduction: Bringing Molecules to Life
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/18
Play
Full screen (f)
Deck 12: Chemical Reactions, Oxidation, and Reduction: Bringing Molecules to Life
1
Which of the following species behave as nucleophiles? Select all that apply.
A) Cl-
B) H+
C) OH
D) CH2CH2
E) NO2+
A) Cl-
B) H+
C) OH
D) CH2CH2
E) NO2+
A,C,D
2
Which of the following species behave as electrophiles? Select all that apply.
A) CN-
B) CH3CH2+
C) H+
D) H2O
E) CHCH
A) CN-
B) CH3CH2+
C) H+
D) H2O
E) CHCH
B,C
3
The stoichiometry of a reaction tells us which of the following?
A) The rate at which a reaction occurs.
B) The amount of energy liberated during the course of a reaction.
C) The number of steps from which an overall reaction, from reactants to products, is formed.
D) The amount of energy required to initiate a reaction.
E) The relative quantities of reactants and products associated with a particular reaction.
A) The rate at which a reaction occurs.
B) The amount of energy liberated during the course of a reaction.
C) The number of steps from which an overall reaction, from reactants to products, is formed.
D) The amount of energy required to initiate a reaction.
E) The relative quantities of reactants and products associated with a particular reaction.
E
4
Which of the following statements about chemical reactions are true? Select all that apply.
A) Chemical reactions involve the breaking of bonds between atoms in molecules, and the formation of new bonds between different groups of atoms.
B) During chemical reactions, valence electrons always move as pairs.
C) The reactants associated with a particular reaction react in different relative quantities depending on the temperature at which the reaction is being performed.
D) Chemical reactions always result in full valence shells being maintained.
E) Chemical reactions require the movement of valence electrons.
A) Chemical reactions involve the breaking of bonds between atoms in molecules, and the formation of new bonds between different groups of atoms.
B) During chemical reactions, valence electrons always move as pairs.
C) The reactants associated with a particular reaction react in different relative quantities depending on the temperature at which the reaction is being performed.
D) Chemical reactions always result in full valence shells being maintained.
E) Chemical reactions require the movement of valence electrons.

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements about valence electrons are false? Select all that apply.
A) When heterolytic cleavage occurs, a pair of valence electrons is distributed unequally between two atoms.
B) We use a curly arrow to denote the movement of a pair of valence electrons.
C) The movement of valence electrons during a chemical reaction is random.
D) During a chemical reaction, valence electrons can move both within and between molecules.
E) The movement of valence electrons during a chemical reaction is described by the reaction scheme.
A) When heterolytic cleavage occurs, a pair of valence electrons is distributed unequally between two atoms.
B) We use a curly arrow to denote the movement of a pair of valence electrons.
C) The movement of valence electrons during a chemical reaction is random.
D) During a chemical reaction, valence electrons can move both within and between molecules.
E) The movement of valence electrons during a chemical reaction is described by the reaction scheme.

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements are true? Select all that apply.
A) An electrophile must possess a partially empty orbital.
B) A nucleophile must be able to donate a pair of valence electrons.
C) A non-bonding pair is not a suitable valence pair for a nucleophile to donate.
D) When a nucleophile donates a pair of valence electrons to an electrophile, an ionic bond is formed.
E) A double bond can act as a nucleophile.
A) An electrophile must possess a partially empty orbital.
B) A nucleophile must be able to donate a pair of valence electrons.
C) A non-bonding pair is not a suitable valence pair for a nucleophile to donate.
D) When a nucleophile donates a pair of valence electrons to an electrophile, an ionic bond is formed.
E) A double bond can act as a nucleophile.

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
7
The cleavage of the H-Br bond is most likely to generate which of the following pairs of species?
A) H+ and Br-
B) H- and Br+
C) H and Br
A) H+ and Br-
B) H- and Br+
C) H and Br

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following bonds are most likely to break homolytically? Select any that apply.
A) H-Br
B) Cl-Cl
C) C-H
D) C-C
E) O-H
A) H-Br
B) Cl-Cl
C) C-H
D) C-C
E) O-H

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
9
Electromagnetic radiation in which of the following regions of the electromagnetic spectrum is most able to generate free radicals?
A) Radio wave
B) Cosmic wave
C) Infrared
D) Visible
E) Ultraviolet
A) Radio wave
B) Cosmic wave
C) Infrared
D) Visible
E) Ultraviolet

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
10
The following reaction represents which stage in a free-radical reaction?
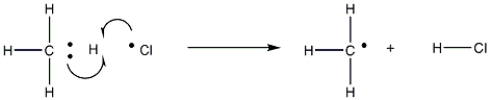
A) Initiation
B) Propagation
C) Termination

A) Initiation
B) Propagation
C) Termination

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements regarding free radicals are true? Select all that apply.
A) Antioxidants catalyse free radical reactions by encouraging the formation of free radicals.
B) A chain reaction involves a sequence of linked propagation reactions.
C) Free-radical reactions involve heterolytic bond cleavage.
D) Free radicals can only be generated from molecules possessing non-polar bonds.
E) The initiation of a free-radical reaction by ultraviolet light is called photolysis.
A) Antioxidants catalyse free radical reactions by encouraging the formation of free radicals.
B) A chain reaction involves a sequence of linked propagation reactions.
C) Free-radical reactions involve heterolytic bond cleavage.
D) Free radicals can only be generated from molecules possessing non-polar bonds.
E) The initiation of a free-radical reaction by ultraviolet light is called photolysis.

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
12
When we forcibly increase the proportion of free radicals entering the termination step of a free-radical reaction we are said to be doing what?
A) Extinguishing the reaction
B) Terminating the reaction
C) Quenching the reaction
D) Exhausting the reaction
E) Expunging the reaction
A) Extinguishing the reaction
B) Terminating the reaction
C) Quenching the reaction
D) Exhausting the reaction
E) Expunging the reaction

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
13
Match the correct terms with the following descriptions.
-A loss of electrons during the course of a reaction
A) Oxidation
B) Reduction
-A loss of electrons during the course of a reaction
A) Oxidation
B) Reduction

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
14
Match the correct terms with the following descriptions.
-A gain of electrons during the course of a reaction
A) Oxidation
B) Reduction
-A gain of electrons during the course of a reaction
A) Oxidation
B) Reduction

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
15
During the process NADH → NAD+ the electron carrier NAD is being reduced.

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
16
Based on the following standard reduction potentials, which of the following species acts as the strongest oxidizing agent?
Zn2+(aq) + 2e- ⇌ Zn(s); E° = -0.34v
Cl2(g) + 2e- ⇌ 2Cl-(aq); E° = 1.36v
Ca2+(aq) + 2e- ⇌ Ca(s); E° = -2.87v
2H+(aq) + 2e- ⇌ H2(g); E° = 0.00v
A) Zn2+(aq)
B) Cl2(g)
C) Ca2+(aq)
D) 2H+(aq)
Zn2+(aq) + 2e- ⇌ Zn(s); E° = -0.34v
Cl2(g) + 2e- ⇌ 2Cl-(aq); E° = 1.36v
Ca2+(aq) + 2e- ⇌ Ca(s); E° = -2.87v
2H+(aq) + 2e- ⇌ H2(g); E° = 0.00v
A) Zn2+(aq)
B) Cl2(g)
C) Ca2+(aq)
D) 2H+(aq)

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
17
The reference electrode against which other standard reduction potentials are measured is which of the following?
A) The platinum electrode
B) The iron electrode
C) The copper electrode
D) The zinc electrode
E) The hydrogen electrode
A) The platinum electrode
B) The iron electrode
C) The copper electrode
D) The zinc electrode
E) The hydrogen electrode

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
18
During the course of a reaction, the oxidation number of oxygen increases from -2 to 0. Is oxygen being oxidized or reduced during this reaction?
A) Oxidized
B) Reduced
A) Oxidized
B) Reduced

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck


