Deck 4: Molecular Interactions: Holding It All Together
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/15
Play
Full screen (f)
Deck 4: Molecular Interactions: Holding It All Together
1
Which three of the following elements is H most likely to be attached to for it to participate in a hydrogen bond? Select the three that apply.
A) N
B) I
C) Br
D) O
E) F
A) N
B) I
C) Br
D) O
E) F
A,D,E
2
Which of the following statements about dispersion forces are true? Select all that apply.
A) Dispersion forces are very short-lived
B) Dispersion forces stabilize hydrophobic interactions.
C) Dispersion forces operate over only very short distances.
D) Dispersion forces can only exist between polar molecules.
E) Dispersion forces are the weakest non-covalent interaction.
A) Dispersion forces are very short-lived
B) Dispersion forces stabilize hydrophobic interactions.
C) Dispersion forces operate over only very short distances.
D) Dispersion forces can only exist between polar molecules.
E) Dispersion forces are the weakest non-covalent interaction.
A,B,C,E
3
Match the following descriptions with the names of the physical processes they represent.
-The transition from solid to liquid
A) Condensation
B) Melting
C) Solidification
D) Sublimation
E) Vaporization
-The transition from solid to liquid
A) Condensation
B) Melting
C) Solidification
D) Sublimation
E) Vaporization
B
4
Match the following descriptions with the names of the physical processes they represent.
-The transition from solid to gas
A) Condensation
B) Melting
C) Solidification
D) Sublimation
E) Vaporization
-The transition from solid to gas
A) Condensation
B) Melting
C) Solidification
D) Sublimation
E) Vaporization

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements about non-covalent interactions are false? Select all that apply.
A) Non-covalent interactions only operate between separate molecules.
B) Non-covalent interactions are primarily electrostatic in nature.
C) Non-covalent interactions only operate over short distances.
D) The weakest non-covalent interaction is the ionic force.
E) The strength of a non-covalent interaction increases as the distance between two molecules increases
A) Non-covalent interactions only operate between separate molecules.
B) Non-covalent interactions are primarily electrostatic in nature.
C) Non-covalent interactions only operate over short distances.
D) The weakest non-covalent interaction is the ionic force.
E) The strength of a non-covalent interaction increases as the distance between two molecules increases

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following molecules are polar? Select all that apply.
A)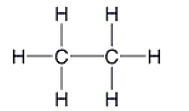
B)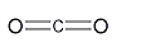
C)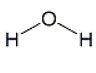
D)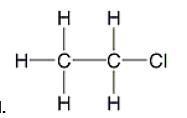
E)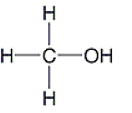
A)

B)
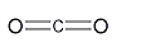
C)

D)

E)


Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
7
Hydrogen bonds are an example of which of the following types of molecular interaction?
A) Salt bridge
B) Ionic force
C) Dispersion force
D) Permanent dipolar interaction
E) Covalent bond
A) Salt bridge
B) Ionic force
C) Dispersion force
D) Permanent dipolar interaction
E) Covalent bond

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is the name given to the molecular force that operates between oppositely-charged amino acid side chains in a protein?
A) van der Waals interaction
B) Ionic bond
C) Salt bridge
D) Dispersion force
E) Peptide bond
A) van der Waals interaction
B) Ionic bond
C) Salt bridge
D) Dispersion force
E) Peptide bond

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following are characteristic of non-polar molecules? Select all that apply.
A) They do not experience dispersion forces.
B) They have relatively low melting and boiling points.
C) Their electrons are distributed unevenly.
D) They are hydrophobic.
E) They never contain polar bonds.
A) They do not experience dispersion forces.
B) They have relatively low melting and boiling points.
C) Their electrons are distributed unevenly.
D) They are hydrophobic.
E) They never contain polar bonds.

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
10
Rank the following physical phases in order of the extent of the non-covalent forces they feature, with 1 being the greatest and 3 being the lowest.

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements about polar molecules are true? Select all that apply.
A) Polar molecules are hydrophilic.
B) Polar molecules have lower melting and boiling points than non-polar molecules.
C) Polar molecules experience more non-covalent forces than non-polar molecules.
D) Polar molecules exhibit an uneven distribution of electrons.
E) Polar molecules do not experience dipolar interactions.
A) Polar molecules are hydrophilic.
B) Polar molecules have lower melting and boiling points than non-polar molecules.
C) Polar molecules experience more non-covalent forces than non-polar molecules.
D) Polar molecules exhibit an uneven distribution of electrons.
E) Polar molecules do not experience dipolar interactions.

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following does not contribute to the van der Waals interaction?
A) Hydrogen bond
B) Dispersion force
C) Steric repulsion
D) Permanent dipolar interaction
A) Hydrogen bond
B) Dispersion force
C) Steric repulsion
D) Permanent dipolar interaction

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements about hydrogen bonds are true? Select all that apply.
A) Hydrogen bonds operate between amino acid side chains in a polypeptide.
B) Hydrogen bonds operate between neighbouring phosphate groups along the backbone of a DNA strand.
C) Hydrogen bonds operate between different regions of the peptide backbone in a polypeptide.
D) Hydrogen bonds do not operate between different regions of an RNA molecule.
E) Hydrogen bonds may operate between the bases of neighbouring DNA strands.
A) Hydrogen bonds operate between amino acid side chains in a polypeptide.
B) Hydrogen bonds operate between neighbouring phosphate groups along the backbone of a DNA strand.
C) Hydrogen bonds operate between different regions of the peptide backbone in a polypeptide.
D) Hydrogen bonds do not operate between different regions of an RNA molecule.
E) Hydrogen bonds may operate between the bases of neighbouring DNA strands.

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
14
Non-covalent interactions are more sensitive to an increase in temperature than covalent interactions.

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
15
Look at this figure of a hydrogen bond. Which entity - X or Y - is acting as the hydrogen bond donor?
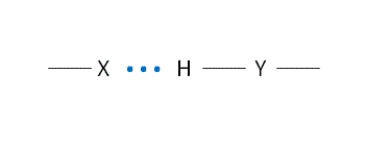
A) X
B) Y
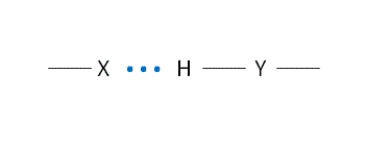
A) X
B) Y

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck


