Deck 2: Atoms: The Foundations of Life
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/15
Play
Full screen (f)
Deck 2: Atoms: The Foundations of Life
1
An atom's nucleus contains which of the following particles? Select all that apply.
A) Ions
B) Protons
C) Electrons
D) Neutrons
A) Ions
B) Protons
C) Electrons
D) Neutrons
B,D
2
An oxygen ion carries what electrical charge?
A) -1
B) +1
C) +2
D) No charge
E) -2
A) -1
B) +1
C) +2
D) No charge
E) -2
E
3
Which of the following is not an element? Select all that apply.
A) Oxygen
B) Water
C) Nitrogen
D) Carbon dioxide
A) Oxygen
B) Water
C) Nitrogen
D) Carbon dioxide
B,D
4
Match the following particles to the electrical charges they carry.
-Proton
A) Positive
B) Negative
C) Neutral
-Proton
A) Positive
B) Negative
C) Neutral

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
5
Match the following particles to the electrical charges they carry.
-Electron
A) Positive
B) Negative
C) Neutral
-Electron
A) Positive
B) Negative
C) Neutral

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
6
Match the following particles to the electrical charges they carry.
-Neutron
A) Positive
B) Negative
C) Neutral
-Neutron
A) Positive
B) Negative
C) Neutral

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
7
An element's mass number tells us which of the following?
A) The number of protons in an atom of that element.
B) The number of protons + electrons in an atom of that element.
C) The number of electrons in an atom of that element.
D) The number of protons + neutrons in an atom of that element.
E) The number of neutrons in an atom of that element.
A) The number of protons in an atom of that element.
B) The number of protons + electrons in an atom of that element.
C) The number of electrons in an atom of that element.
D) The number of protons + neutrons in an atom of that element.
E) The number of neutrons in an atom of that element.

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
8
An atom of fluorine has an atomic number of 9 and a mass number of 19. Which of the following statements is correct?
A) An atom of fluorine has 9 protons, 9 electrons, and 10 neutrons.
B) An atom of fluorine has 9 protons, 9 electrons, and 9 neutrons.
C) An atom of fluorine has 9 protons, 10 electrons, and 9 neutrons.
D) An atom of fluorine has 10 protons, 9 electrons, and 9 neutrons.
E) An atom of fluorine has 9 protons, 9 electrons, and 19 neutrons.
A) An atom of fluorine has 9 protons, 9 electrons, and 10 neutrons.
B) An atom of fluorine has 9 protons, 9 electrons, and 9 neutrons.
C) An atom of fluorine has 9 protons, 10 electrons, and 9 neutrons.
D) An atom of fluorine has 10 protons, 9 electrons, and 9 neutrons.
E) An atom of fluorine has 9 protons, 9 electrons, and 19 neutrons.

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following statements is correct?
A) The three isotopes of carbon contain the same number of protons and neutrons, but different numbers of electrons.
B) The three isotopes of carbon contain the same number of protons and electrons, but different numbers of neutrons.
C) The three isotopes of carbon contain the same number of electrons and neutrons, but different numbers of protons.
D) The three isotopes of carbon contain the same number of protons, neutrons, and electrons.
E) The three isotopes of carbon contain the same number of protons, but different numbers of electrons and neutrons.
A) The three isotopes of carbon contain the same number of protons and neutrons, but different numbers of electrons.
B) The three isotopes of carbon contain the same number of protons and electrons, but different numbers of neutrons.
C) The three isotopes of carbon contain the same number of electrons and neutrons, but different numbers of protons.
D) The three isotopes of carbon contain the same number of protons, neutrons, and electrons.
E) The three isotopes of carbon contain the same number of protons, but different numbers of electrons and neutrons.

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
10
The 3d sub-shell comprises how many orbitals?
A) One
B) Two
C) Three
D) Four
E) Five
A) One
B) Two
C) Three
D) Four
E) Five

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
11
Rank the following sub-shells in order of energy, with 1 being the lowest-energy, and 4 being the highest-energy

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
12
Shell 2 can hold a maximum of how many electrons?
A) 2
B) 4
C) 6
D) 8
E) 10
A) 2
B) 4
C) 6
D) 8
E) 10

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
13
Noting that chlorine has an atomic number of 17, which of the following statements are false? Select any that apply.
A) An atom of chlorine has 17 electrons.
B) Chlorine's valence shell is shell 2.
C) The electronic configuration for chlorine is 1s2 2s2 2p6 3s2 3p5
D) Chlorine must gain 1 electron to achieve a full valence shell.
E) The chloride ion carries a charge of +1
A) An atom of chlorine has 17 electrons.
B) Chlorine's valence shell is shell 2.
C) The electronic configuration for chlorine is 1s2 2s2 2p6 3s2 3p5
D) Chlorine must gain 1 electron to achieve a full valence shell.
E) The chloride ion carries a charge of +1

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
14
Nitrogen has an atomic number of 7. Which of the following correctly depicts the electronic configuration of an atom of nitrogen?
A)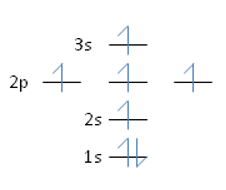
B)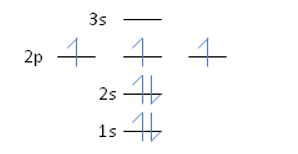
C)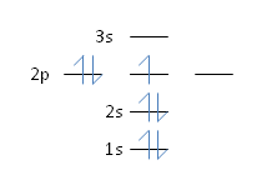
D)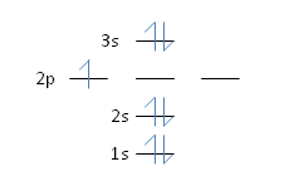
A)
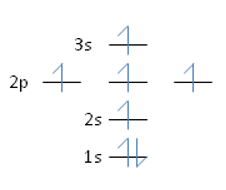
B)
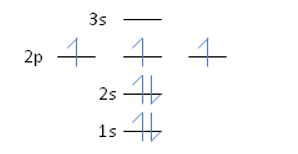
C)
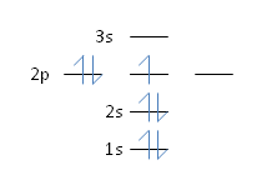
D)
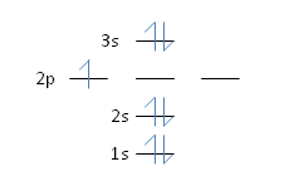

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck
15
Rank the following elements according to how readily they will form anions, with 1 = least readily, and 4 = most readily.

Unlock Deck
Unlock for access to all 15 flashcards in this deck.
Unlock Deck
k this deck


