Deck 5: The Nature of Light
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/91
Play
Full screen (f)
Deck 5: The Nature of Light
1
No matter how close or how far Galileo was from his assistant, he always measured roughly the same time in performing the lantern experiment. Just what time was Galileo measuring?
A)the time for light to travel from his assistant to himself
B)the round-trip time for light to travel between Galileo and his assistant
C)his own reaction time-that is, the time for him to open his shutter after observing his assistant's light
D)the combined reaction time of Galileo and his assistant
A)the time for light to travel from his assistant to himself
B)the round-trip time for light to travel between Galileo and his assistant
C)his own reaction time-that is, the time for him to open his shutter after observing his assistant's light
D)the combined reaction time of Galileo and his assistant
D
2
The first experiment in which the speed of light was measured precisely involved:
A)timing eclipses of Jupiter's satellites, which appeared to occur later when Earth was farther from Jupiter.
B)measuring how long it took the light from stars located at different distances to reach Earth.
C)reflecting light from a mirror rotating at a known speed and measuring the angle of deflection of the light beam.
D)opening a shutter on a lantern on a hilltop and measuring the time taken for light from an assistant's shuttered lantern to return.
A)timing eclipses of Jupiter's satellites, which appeared to occur later when Earth was farther from Jupiter.
B)measuring how long it took the light from stars located at different distances to reach Earth.
C)reflecting light from a mirror rotating at a known speed and measuring the angle of deflection of the light beam.
D)opening a shutter on a lantern on a hilltop and measuring the time taken for light from an assistant's shuttered lantern to return.
C
3
The first experiment to measure the speed of light accurately was made by the:
A)French physicists, Fizeau and Foucault.
B)Danish astronomer, Rømer.
C)Italian scientist, Galileo.
D)German/American physicist, Einstein.
A)French physicists, Fizeau and Foucault.
B)Danish astronomer, Rømer.
C)Italian scientist, Galileo.
D)German/American physicist, Einstein.
A
4
The speed of light:
A)was first accurately determined by Galileo in an experiment involving lanterns and shutters.
B)can be determined by observing the motions of the moons of Jupiter.
C)was not determined until the advent of lasers in 1960.
D)is too high to measure and consequently is still unknown.
A)was first accurately determined by Galileo in an experiment involving lanterns and shutters.
B)can be determined by observing the motions of the moons of Jupiter.
C)was not determined until the advent of lasers in 1960.
D)is too high to measure and consequently is still unknown.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
5
Rømer calculated the speed of light by observing the large moons of Jupiter being eclipsed by Jupiter while that planet was at opposition and while it was at superior conjunction. How large is the difference in the Jupiter-Earth distance when Jupiter is at opposition and when it is at superior conjunction?
A)1 au
B)2 au
C)1 ly
D)0 au
A)1 au
B)2 au
C)1 ly
D)0 au

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
6
Earth is about 150,000,000 km from the Sun. How long did it take light to cross Earth's orbit in Rømer's experimental determination of the speed of light?
A)about 8 seconds
B)about 16 seconds
C)about 8 minutes
D)about 16 minutes
A)about 8 seconds
B)about 16 seconds
C)about 8 minutes
D)about 16 minutes

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
7
Assuming that Uranus was at opposition to Earth when Voyager II sent back its historic (and magnificent) pictures from Uranus in January 1986, how long did those signals take to arrive after transmission by Voyager? The average distance of Neptune from the Sun is about .
A)9066.7 min
B)151.1 hrs
C)5 hrs 2 min
D)2 hrs 31 min
A)9066.7 min
B)151.1 hrs
C)5 hrs 2 min
D)2 hrs 31 min

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
8
As of 2018, Voyager 1 is in interstellar space, about 13 billion miles from Earth. How long does it take data transmitted by Voyager 1 to reach the Deep Space Network on Earth?
A)about 90 min
B)about 19 hrs
C)3 days
D)2 weeks
A)about 90 min
B)about 19 hrs
C)3 days
D)2 weeks

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
9
White light passes through a prism and separates into a spectrum of colors. A second prism is placed so that only the green light from the first prism falls upon it. After passing through this second prism the light will be:
A)white.
B)green.
C)ultraviolet.
D)infrared.
A)white.
B)green.
C)ultraviolet.
D)infrared.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
10
The rear brake lights on a car emit white light but are covered with plastic that allows only pure red light to pass through. What color of plastic would the red light have to pass through in order to emerge as green light?
A)white
B)green
C)blue
D)There is no color plastic that will turn pure red light into green.
A)white
B)green
C)blue
D)There is no color plastic that will turn pure red light into green.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
11
Light of a single wavelength falls on a screen with two narrow, closely spaced slits. On a second screen, a short distance beyond the first:
A)nothing will be seen since light cannot pass through narrow slits.
B)two bright lines corresponding to the two slits will be observed.
C)a totally random mixture of light and dark will be seen.
D)a series of bright lines with dark spaces in between will be seen.
A)nothing will be seen since light cannot pass through narrow slits.
B)two bright lines corresponding to the two slits will be observed.
C)a totally random mixture of light and dark will be seen.
D)a series of bright lines with dark spaces in between will be seen.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
12
Around 1801, Thomas Young in England showed that light behaves as a wave by:
A)deriving a set of mathematical equations that described electromagnetic waves that could have different wavelengths.
B)shining light through two closely spaced slits and observing the resulting pattern of light on a white screen.
C)reflecting light from a rotating mirror and measuring the deflection in different directions.
D)shining light through a glass prism and observing the resulting pattern of colors on a white screen.
A)deriving a set of mathematical equations that described electromagnetic waves that could have different wavelengths.
B)shining light through two closely spaced slits and observing the resulting pattern of light on a white screen.
C)reflecting light from a rotating mirror and measuring the deflection in different directions.
D)shining light through a glass prism and observing the resulting pattern of colors on a white screen.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
13
Who was the first person to suggest that light is made of particles?
A)Isaac Newton
B)Christiaan Huygens
C)Thomas Young
D)James Clerk Maxwell
A)Isaac Newton
B)Christiaan Huygens
C)Thomas Young
D)James Clerk Maxwell

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
14
In order of increasing frequency, some of the colors that the visible spectrum contains are:
A)blue, red, and yellow.
B)red, blue, and yellow.
C)red, yellow, and blue.
D)blue, yellow, and red.
A)blue, red, and yellow.
B)red, blue, and yellow.
C)red, yellow, and blue.
D)blue, yellow, and red.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
15
The diameter of your finger is 1 or 2 cm. What type of electromagnetic wave has a wavelength this size?
A)ultraviolet
B)visible
C)microwave
D)radio
A)ultraviolet
B)visible
C)microwave
D)radio

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
16
How does the frequency at the red end of the visible spectrum compare with the frequency at the blue-violet end of the visible spectrum?
A)It is about twice as big.
B)It is about half as big.
C)It is several orders of magnitude smaller.
D)It is several orders of magnitude larger.
A)It is about twice as big.
B)It is about half as big.
C)It is several orders of magnitude smaller.
D)It is several orders of magnitude larger.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
17
How does the wavelength at the red end of the visible spectrum compare with the wavelength at the blue-violet end of the visible spectrum?
A)It is about twice as big.
B)It is about half as big.
C)It is several orders of magnitude smaller.
D)It is several orders of magnitude larger.
A)It is about twice as big.
B)It is about half as big.
C)It is several orders of magnitude smaller.
D)It is several orders of magnitude larger.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
18
What is the wavelength of radio waves from the FM radio station KTYD, which operates at the frequency 99.9 MHz?
A)99.9 m
B)3.0 m
C)300 m
D)33.3 m
A)99.9 m
B)3.0 m
C)300 m
D)33.3 m

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
19
Choose the correct sequence of electromagnetic radiations, in order of increasing wavelengths.
A)radio, IR, visible, UV
B)UV, visible, radio, IR
C)UV, visible, IR, radio
D)visible, UV, IR, radio
A)radio, IR, visible, UV
B)UV, visible, radio, IR
C)UV, visible, IR, radio
D)visible, UV, IR, radio

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
20
In terms of wavelengths, gamma rays are:
A)shorter than x rays.
B)between radio and infrared waves.
C)between x rays and ultraviolet waves.
D)longer than visible light.
A)shorter than x rays.
B)between radio and infrared waves.
C)between x rays and ultraviolet waves.
D)longer than visible light.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
21
What is one fundamental difference between x rays and radio waves?
A)They always come from different sources.
B)Their wavelengths are very different.
C)Radio waves are always wavelike, while x rays always behave like particles.
D)Their speeds in outer space are different.
A)They always come from different sources.
B)Their wavelengths are very different.
C)Radio waves are always wavelike, while x rays always behave like particles.
D)Their speeds in outer space are different.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
22
What is the wavelength of electromagnetic radiation whose frequency is 106 cycles per second (106 Hz or 1000 kHz, the frequency of ordinary AM radio)?
A)3 mm
B)3 cm
C)3 m
D)300 m
A)3 mm
B)3 cm
C)3 m
D)300 m

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
23
In a radio wave transmitter (such as that used by a radio or TV station), when the frequency of the signals is increased, the:
A)wavelength is decreased.
B)speed of transmission of the waves is increased.
C)wavelength and speed of transmission both increase.
D)wavelength remains constant.
A)wavelength is decreased.
B)speed of transmission of the waves is increased.
C)wavelength and speed of transmission both increase.
D)wavelength remains constant.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
24
Which one of these scientists was the last to perform his investigation of electromagnetic radiation?
A)Newton (the prism experiment)
B)Hertz (production of radio waves)
C)Young (two-slit interference experiment)
D)Huygens (wave theory of light)
A)Newton (the prism experiment)
B)Hertz (production of radio waves)
C)Young (two-slit interference experiment)
D)Huygens (wave theory of light)

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
25
The temperature of a gas cloud in space is directly related to and representative of the:
A)number of atomic collisions per second within the cloud.
B)average speed of its atoms.
C)density of the cloud.
D)color of the cloud.
A)number of atomic collisions per second within the cloud.
B)average speed of its atoms.
C)density of the cloud.
D)color of the cloud.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
26
To what physical parameter is the temperature of a thin gas most closely related?
A)average number of collisions per second between molecules
B)pressure of the gas
C)average speed of the molecules
D)mean mass per unit volume, or density, of the gas
A)average number of collisions per second between molecules
B)pressure of the gas
C)average speed of the molecules
D)mean mass per unit volume, or density, of the gas

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
27
At absolute zero temperature, which of the following conditions holds?
A)Electrons stop moving around the nuclei of atoms.
B)The motion of atoms ceases.
C)Electrons in all atoms move to their ground states.
D)The motion of atoms becomes the minimum possible (but not zero).
A)Electrons stop moving around the nuclei of atoms.
B)The motion of atoms ceases.
C)Electrons in all atoms move to their ground states.
D)The motion of atoms becomes the minimum possible (but not zero).

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
28
An example of an object that emits no radiation at all is:
A)an object with the temperature of outer space.
B)a blackbody.
C)an object made of ice.
D)an object at a temperature of 0 K.
A)an object with the temperature of outer space.
B)a blackbody.
C)an object made of ice.
D)an object at a temperature of 0 K.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
29
Two physicists, one in Australia, the other in the United States, find that each has constructed an ideal blackbody in the laboratory. The two blackbodies are made from very different materials. Without conducting tests, they know that the radiation emitted by these two objects will be:
A)different because the amount of light falling on them is likely to be different in the two laboratories.
B)identical to each other if the blackbodies have the same size, even if their temperatures are different.
C)different from each other because of the difference in materials.
D)identical to each other if the blackbodies are at the same temperature but not otherwise.
A)different because the amount of light falling on them is likely to be different in the two laboratories.
B)identical to each other if the blackbodies have the same size, even if their temperatures are different.
C)different from each other because of the difference in materials.
D)identical to each other if the blackbodies are at the same temperature but not otherwise.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
30
Figure 5-12 in Universe, 11th ed., shows that a blackbody with a temperature of 3000 K emits radiation that peaks at a wavelength much longer than wavelengths in the visible part of the spectrum. This means that:
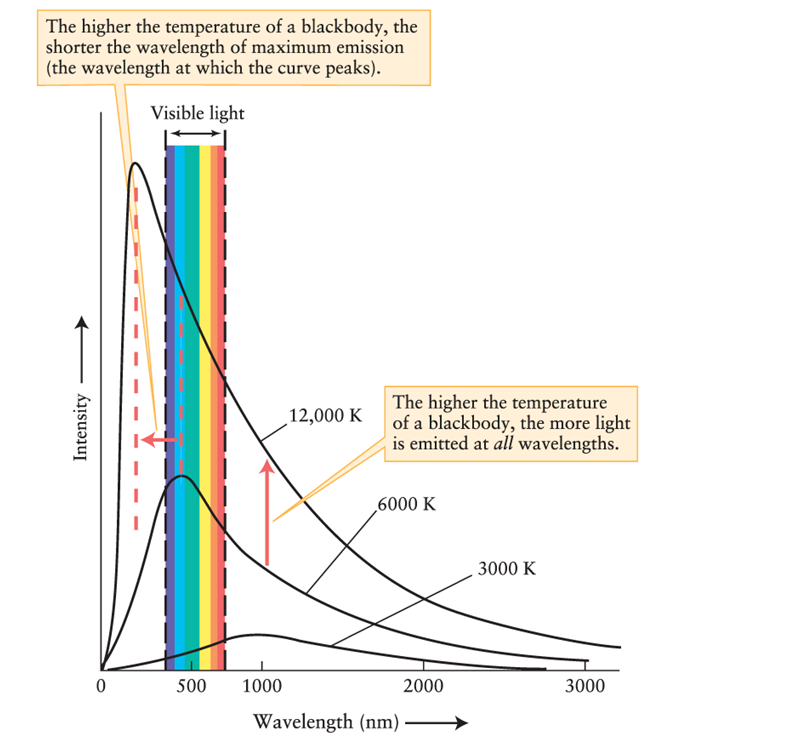
A)the object is not visible but might be detected with equipment sensitive to nonvisible radiation.
B)the object, like all blackbodies, emits no radiation.
C)the object emits visible radiation, but not as intensely as at longer wavelengths.
D)no visible radiation is emitted, but visible radiation would be emitted if the temperature of the object were increased.

A)the object is not visible but might be detected with equipment sensitive to nonvisible radiation.
B)the object, like all blackbodies, emits no radiation.
C)the object emits visible radiation, but not as intensely as at longer wavelengths.
D)no visible radiation is emitted, but visible radiation would be emitted if the temperature of the object were increased.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
31
Compare the spectrum emitted from a blackbody at 12,000 K and at 3000 K, using Figure 5-12 of Universe, 11th ed. Where in the spectrum does the object emit more radiation when it is cooler than when it is warmer?
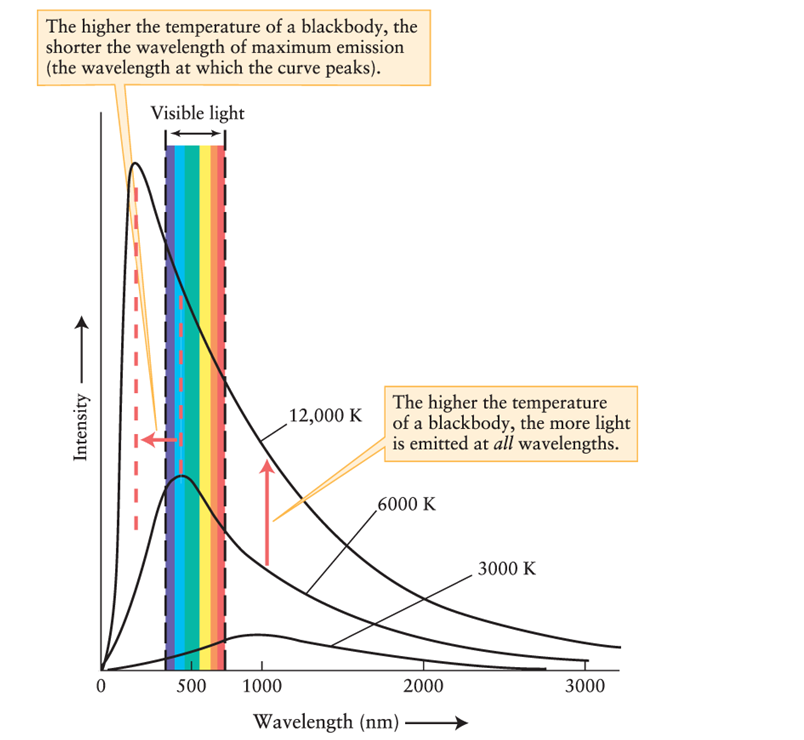
A)ultraviolet
B)visible
C)infrared
D)nowhere

A)ultraviolet
B)visible
C)infrared
D)nowhere

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
32
Wien's law, relating the peak wavelength λmax of light emitted by a dense object to its temperature T, can be represented by:
A)λmax = constant × T 4.
B)λmax T = constant.
C)λmax = constant/T 2.
D)λmax/T = constant.
A)λmax = constant × T 4.
B)λmax T = constant.
C)λmax = constant/T 2.
D)λmax/T = constant.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
33
As a new star evolves from cool dust and gas to a hot star, the peak wavelength of its spectrum of electromagnetic radiation:
A)changes from the ultraviolet to the visible range.
B)changes from the infrared to the visible wavelengths.
C)increases from the visible to infrared wavelengths.
D)remains the same.
A)changes from the ultraviolet to the visible range.
B)changes from the infrared to the visible wavelengths.
C)increases from the visible to infrared wavelengths.
D)remains the same.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
34
Using Wien's law, which relates the peak wavelength λmax, (1 μm = 10-6 m) emitted by a body to its temperature T, what is the peak wavelength of electromagnetic radiation emitted by a piece of iron that is just melting (1538°C)? (See Box 5-2 and Figure 5-7 of Universe, 11th ed.)
A)16 μm, intermediate infrared
B)1.89 μm, near infrared
C)1.04 μm, very near infrared
D)1.6 μm, near infrared
A)16 μm, intermediate infrared
B)1.89 μm, near infrared
C)1.04 μm, very near infrared
D)1.6 μm, near infrared

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
35
You are asked to design a detection system for human beings (or animals) in darkness, using infrared detection. If human beings are at a temperature of about 310 K, what would need to be the wavelength of peak sensitivity of your equipment or cameras (1 μm = 10-6 m)? (Hint: Use Wien's law.)
A)9.35 μm
B)0.935 μm
C)0.00094 μm or 0.94 nm
D)90 μm
A)9.35 μm
B)0.935 μm
C)0.00094 μm or 0.94 nm
D)90 μm

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
36
Using Wien's law, what is the approximate peak wavelength of radiation emitted by (live) human beings, who are (normally) at a temperature of about 310 K (1 μm = 10-6 m)?
A)9.4 μm
B)3.1 μm
C)94 μm
D)0.94 μm
A)9.4 μm
B)3.1 μm
C)94 μm
D)0.94 μm

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
37
The wavelength of the peak in the Sun's output spectrum is about 500 nm. If the Sun's surface temperature doubled, what would this peak wavelength become?
A)250 nm
B)500 nm
C)750 nm
D)1000 nm
A)250 nm
B)500 nm
C)750 nm
D)1000 nm

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
38
An astronomer wants to know whether a particular star has a greater surface temperature than the Sun. What does she need to know about this star to determine its temperature?
A)the wavelength at the peak of its spectrum
B)its distance from the Sun
C)its size
D)its age
A)the wavelength at the peak of its spectrum
B)its distance from the Sun
C)its size
D)its age

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
39
The total energy flux F of radiation emitted per unit area by a blackbody (e.g., star) is related to its temperature T and a constant σ by which equation?
A)F = σT 4
B)FT 4 = σ
C)F 4 = σT
D)F = σ /T
A)F = σT 4
B)FT 4 = σ
C)F 4 = σT
D)F = σ /T

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
40
When a solid body is heated to a temperature T, the total radiated energy flux F from this body per second per unit area is given by (where σ is a constant):
A)F = σT.
B)F = σ/T 2.
C)F = σT 4.
D)FT = σ.
A)F = σT.
B)F = σ/T 2.
C)F = σT 4.
D)FT = σ.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
41
The laws governing the energy flux F and wavelength of maximum intensity λmax of emitted radiation from a hot, dense body whose temperature is T are given by (where σ and a are constants):
A)F = σT 2, λmax T = a.
B)F = σT 4, λmax T = a.
C)F = σT, λmax = a/T 4.
D)F = σT 4, λmax = aT.
A)F = σT 2, λmax T = a.
B)F = σT 4, λmax T = a.
C)F = σT, λmax = a/T 4.
D)F = σT 4, λmax = aT.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
42
A piece of iron is heated from 400 K to 800 K (127°C to 527°C). By what factor will the total energy per second emitted by this iron increase?
A)2
B)296.5
C)4
D)16
A)2
B)296.5
C)4
D)16

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
43
The energy flux arriving at Earth from the Sun is known as the solar constant and has a value of 1.37 × 103 watts per square meter. Assuming that the atmosphere absorbs 50% of the energy and that a 5-m2 roof collector is available to collect energy with a 30% efficiency, how much of this solar energy would then be available for use in the house for water or house heating, etc.? (1 KW = 1 kilowatt = 1000 W)
A)about 1 KW
B)about 46 KW
C)about 1 W
D)about 10 KW
A)about 1 KW
B)about 46 KW
C)about 1 W
D)about 10 KW

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
44
The Stefan-Boltzmann law relating energy per unit area F emitted by an object to its temperature T, F = σT 4, is obeyed ideally by what type of object?
A)only hot gases, whose atoms emit and absorb only specific colors (e.g., neon tubes)
B)all objects, whatever their color or reflective properties
C)a red-colored object that absorbs blue light but reflects red light
D)a blackbody, a perfect absorber and emitter of energy at all wavelengths
A)only hot gases, whose atoms emit and absorb only specific colors (e.g., neon tubes)
B)all objects, whatever their color or reflective properties
C)a red-colored object that absorbs blue light but reflects red light
D)a blackbody, a perfect absorber and emitter of energy at all wavelengths

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
45
The radius of the Sun is about 1/200 of an astronomical unit. What happens to the flux of solar energy as it travels from the Sun's surface to Earth?
A)It remains constant.
B)It increases by a factor of 200.
C)It decreases by a factor of 200.
D)It decreases by a factor of (200)2 = 40,000.
A)It remains constant.
B)It increases by a factor of 200.
C)It decreases by a factor of 200.
D)It decreases by a factor of (200)2 = 40,000.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
46
Can a cooler object ever emit more radiation than a warmer object?
A)No.
B)Yes, if the two have the same surface area.
C)Yes, if the cooler object has an area Acooler that meets the requirement Tcooler Acooler > Thot Ahot.
D)Yes, if the cooler object has an area Acooler that meets the requirement (Tcooler )4Acooler > (Thot )4 Ahot.
A)No.
B)Yes, if the two have the same surface area.
C)Yes, if the cooler object has an area Acooler that meets the requirement Tcooler Acooler > Thot Ahot.
D)Yes, if the cooler object has an area Acooler that meets the requirement (Tcooler )4Acooler > (Thot )4 Ahot.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
47
The Sun's surface has a temperature of 5800 K. What will be the peak wavelength of the spectrum of a star that emits twice the Sun's flux of energy?
A)2.1 × 10-7 m
B)4.0 × 10-7 m
C)5.0 × 10-7 m
D)1.0 × 10-6 m
A)2.1 × 10-7 m
B)4.0 × 10-7 m
C)5.0 × 10-7 m
D)1.0 × 10-6 m

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
48
Proxima Centauri, our Sun's nearest stellar neighbor, has a surface temperature of about 3300 K. At what wavelength does Proxima Centauri emit its most intense radiation?
A)about 200 nm
B)about 400 nm
C)about 600 nm
D)about 900 nm
A)about 200 nm
B)about 400 nm
C)about 600 nm
D)about 900 nm

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
49
Proxima Centauri, our Sun's nearest stellar neighbor, has a surface temperature of about 3300 K. What color will this star appear to the naked eye?
A)red
B)yellow
C)white
D)blue
A)red
B)yellow
C)white
D)blue

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
50
In its interaction with matter, light behaves:
A)only as waves.
B)alternatively as particles or as waves, switching its properties about once every second.
C)as both waves and particles, depending on the type of interaction.
D)only as small particles, photons.
A)only as waves.
B)alternatively as particles or as waves, switching its properties about once every second.
C)as both waves and particles, depending on the type of interaction.
D)only as small particles, photons.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
51
A photon is:
A)a positively charged particle in the atomic nucleus.
B)an element with a high atomic number.
C)a particle that orbits the nucleus of an atom.
D)a bundle of pure energy.
A)a positively charged particle in the atomic nucleus.
B)an element with a high atomic number.
C)a particle that orbits the nucleus of an atom.
D)a bundle of pure energy.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
52
In comparing photons of different wavelengths, we find that the energy carried by a photon:
A)increases as the wavelength increases up to a wavelength equal to λmax, then decreases again.
B)does not depend on its wavelength.
C)is larger if the wavelength is shorter.
D)is larger if the wavelength is longer.
A)increases as the wavelength increases up to a wavelength equal to λmax, then decreases again.
B)does not depend on its wavelength.
C)is larger if the wavelength is shorter.
D)is larger if the wavelength is longer.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
53
The energy of a photon of x rays, compared to the energy of a photon of visible light, is:
A)about the same.
B)much lower.
C)variable and can be higher or lower under certain circumstances and in certain positions in the universe.
D)much higher.
A)about the same.
B)much lower.
C)variable and can be higher or lower under certain circumstances and in certain positions in the universe.
D)much higher.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
54
The energy E of a photon or quantum of electromagnetic radiation is related to the wavelength λ of the radiation by what relation (h = Planck's constant)?
A)E = hc/λ
B)E = hλ
C)E = hcλ
D)E = h/cλ
A)E = hc/λ
B)E = hλ
C)E = hcλ
D)E = h/cλ

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
55
The human eye is most sensitive to light with a wavelength near 550 nm. To what photon energy is the human eye most sensitive?
A)2.49 eV
B)3.61 × 10-19 eV
C)2.25 eV
D)1.83 eV
A)2.49 eV
B)3.61 × 10-19 eV
C)2.25 eV
D)1.83 eV

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
56
What is the energy in eV of a photon with the wavelength of Lyman Lα at 121.5 nm in the ultraviolet range?
A)1030 eV
B)1.51 × 10-4 eV
C)1.02 × 10-8 eV
D)10.2 eV
A)1030 eV
B)1.51 × 10-4 eV
C)1.02 × 10-8 eV
D)10.2 eV

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
57
What is the wavelength of the radiation whose photons have 1 eV of energy?
A)8.1 × 10-4 nm
B)1240 nm in the infrared range
C)1.24 × 10-6 nm, gamma rays
D)124 nm in the ultraviolet range
A)8.1 × 10-4 nm
B)1240 nm in the infrared range
C)1.24 × 10-6 nm, gamma rays
D)124 nm in the ultraviolet range

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
58
What is the energy in electron volts of a photon whose wavelength is the diameter of a typical atom, about 0.1 nm?
A)12.4 keV, or 12,400 eV
B)1.24 × 10-7 eV
C)1.24 keV, or 1240 eV
D)8.061 MeV, or 8,061,000 eV
A)12.4 keV, or 12,400 eV
B)1.24 × 10-7 eV
C)1.24 keV, or 1240 eV
D)8.061 MeV, or 8,061,000 eV

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
59
The dark absorption lines in the solar spectrum are caused by absorption:
A)of sunlight in a layer of pure hydrogen gas overlying the solar surface.
B)of sunlight in a cooler layer of gas overlying the hot solar surface.
C)entirely by atoms and molecules in Earth's cool atmosphere.
D)of sunlight in a hotter layer of gas overlying the cooler solar surface.
A)of sunlight in a layer of pure hydrogen gas overlying the solar surface.
B)of sunlight in a cooler layer of gas overlying the hot solar surface.
C)entirely by atoms and molecules in Earth's cool atmosphere.
D)of sunlight in a hotter layer of gas overlying the cooler solar surface.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
60
If light from a hot, dense star is viewed through a cool cloud of gas (See Figure 5-17 of Universe, 11th ed.):
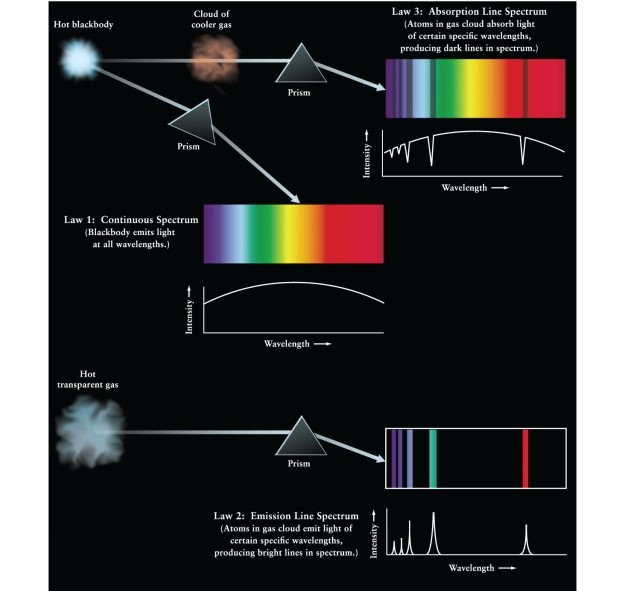
A)the spectrum of the star will still be seen unchanged because the gas cloud is cool.
B)only specific wavelengths of light will be removed from the spectrum.
C)the whole spectrum will be reduced in intensity.
D)the atoms of the gas cloud will add energy to the overall spectrum, producing emission lines at specific wavelengths.

A)the spectrum of the star will still be seen unchanged because the gas cloud is cool.
B)only specific wavelengths of light will be removed from the spectrum.
C)the whole spectrum will be reduced in intensity.
D)the atoms of the gas cloud will add energy to the overall spectrum, producing emission lines at specific wavelengths.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
61
During a dust storm the Sun often appears to redden. This means that the particles of dust:
A)are equal in size to the wavelength of red light.
B)are larger than the wavelengths of visible light.
C)are smaller than the wavelengths of visible light.
D)must be red in color.
A)are equal in size to the wavelength of red light.
B)are larger than the wavelengths of visible light.
C)are smaller than the wavelengths of visible light.
D)must be red in color.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
62
You are looking at a glowing field of hot lava from a satellite high above Earth's atmosphere. Hot lava emits most of its radiation in the infrared, and Earth's atmosphere is not completely transparent to infrared. What kind of spectrum will you see?
A)a flat spectrum, equally intense at short and long wavelengths
B)an emission spectrum
C)an absorption spectrum
D)no spectrum at any wavelength
A)a flat spectrum, equally intense at short and long wavelengths
B)an emission spectrum
C)an absorption spectrum
D)no spectrum at any wavelength

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
63
The overall diameter of a typical atom is about:
A)10-7 m, or 102 nm.
B)10-5 m, or 104 nm.
C)10-14 m, or 10-5 nm.
D)10-10 m, or 0.1 nm.
A)10-7 m, or 102 nm.
B)10-5 m, or 104 nm.
C)10-14 m, or 10-5 nm.
D)10-10 m, or 0.1 nm.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
64
The typical size of an atom is:
A)10-6 m.
B)10-8 m.
C)1 m.
D)10-10 m.
A)10-6 m.
B)10-8 m.
C)1 m.
D)10-10 m.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
65
The mass of a proton is:
A)about twice as large as that of an electron.
B)almost 2000 times greater than that of an electron.
C)about the same as that of an electron.
D)about 1/2000 as large as that of an electron.
A)about twice as large as that of an electron.
B)almost 2000 times greater than that of an electron.
C)about the same as that of an electron.
D)about 1/2000 as large as that of an electron.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
66
The majority of the naturally occurring elements in the periodic table that decay radioactively are:
A)arranged randomly throughout the table, because stability is largely independent of nuclear mass.
B)at the low-mass end of the table, since the nuclear mass is too small to maintain stability.
C)in the center of the table.
D)at the high-mass end of the table, since the large atomic nuclei are unstable.
A)arranged randomly throughout the table, because stability is largely independent of nuclear mass.
B)at the low-mass end of the table, since the nuclear mass is too small to maintain stability.
C)in the center of the table.
D)at the high-mass end of the table, since the large atomic nuclei are unstable.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
67
According to the arrangement of elements in the periodic table, which of the following elements would be expected to have chemical properties most similar to those of nitrogen, N, whose atomic number is 7?
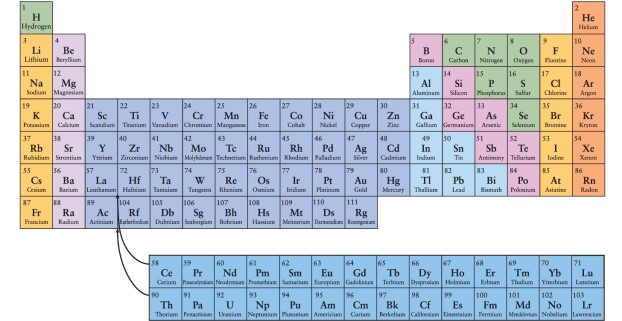
A)oxygen (O, atomic number 8)
B)phosphorous (P, atomic number 15)
C)chlorine (Cl, atomic number 17)
D)carbon (C, atomic number 6)

A)oxygen (O, atomic number 8)
B)phosphorous (P, atomic number 15)
C)chlorine (Cl, atomic number 17)
D)carbon (C, atomic number 6)

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
68
Isotopes of a particular element in the periodic table have which nuclear property in common?
A)same number of neutrons but different numbers of protons
B)same number of neutrons, but different numbers of protons and electrons
C)same total number of protons and neutrons
D)same number of protons but different numbers of neutrons
A)same number of neutrons but different numbers of protons
B)same number of neutrons, but different numbers of protons and electrons
C)same total number of protons and neutrons
D)same number of protons but different numbers of neutrons

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
69
How many neutrons are there in the nucleus of the isotope 18O of oxygen?
A)8
B)18
C)9
D)10
A)8
B)18
C)9
D)10

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
70
How do the spectra of the two isotopes 1H and 2H compare?
A)These isotopes each have one proton, which makes them both hydrogen, so they have identical spectra.
B)The wavelengths for the heavier isotope are very slightly longer than those for the lighter isotope.
C)The wavelengths for the heavier isotope are very slightly shorter than those for the lighter isotope.
D)Since the nuclei are different, the two spectra differ greatly.
A)These isotopes each have one proton, which makes them both hydrogen, so they have identical spectra.
B)The wavelengths for the heavier isotope are very slightly longer than those for the lighter isotope.
C)The wavelengths for the heavier isotope are very slightly shorter than those for the lighter isotope.
D)Since the nuclei are different, the two spectra differ greatly.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
71
One atom of 13C has how many particles?
A)13: 6 protons, 1 neutron, 6 electrons
B)19: 6 protons, 7 neutrons, 6 electrons
C)20: 6 protons, 7 neutrons, 7 electrons
D)39: 13 protons, 13 neutrons, 13 electrons
A)13: 6 protons, 1 neutron, 6 electrons
B)19: 6 protons, 7 neutrons, 6 electrons
C)20: 6 protons, 7 neutrons, 7 electrons
D)39: 13 protons, 13 neutrons, 13 electrons

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
72
The specific sequence of spectral line series emitted by excited hydrogen atoms, in order of increasing wavelength range, is:
A)Balmer, Lyman, Paschen.
B)Lyman, Balmer, Paschen.
C)Lyman, Paschen, Balmer.
D)Paschen, Balmer, Lyman.
A)Balmer, Lyman, Paschen.
B)Lyman, Balmer, Paschen.
C)Lyman, Paschen, Balmer.
D)Paschen, Balmer, Lyman.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
73
The upward Lyman series transitions in hydrogen begin with electrons in the ground state (n = 1), which is the state of most hydrogen atoms. Yet the Balmer series was studied first and was the basis of the early attempts to explain the lines. This is because:
A)upward transitions never occur; only downward transitions occur.
B)the entire Balmer series is in the visible part of the spectrum.
C)the first few transitions (low n) in the Balmer series are in the visible.
D)the last few transitions (high n) in the Balmer series are in the visible.
A)upward transitions never occur; only downward transitions occur.
B)the entire Balmer series is in the visible part of the spectrum.
C)the first few transitions (low n) in the Balmer series are in the visible.
D)the last few transitions (high n) in the Balmer series are in the visible.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
74
Compare two electron transitions in hydrogen: n = 3 → n = 1 and n = 2 → n = 1. Which produces the photon with the higher frequency?
A)n = 3 → n = 1
B)n = 2 → n = 1
C)The frequencies are the same.
D)It is not possible to determine the higher frequency without additional information.
A)n = 3 → n = 1
B)n = 2 → n = 1
C)The frequencies are the same.
D)It is not possible to determine the higher frequency without additional information.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
75
When a hydrogen atom is excited, it emits visible radiation. This radiation is predominantly in the:
A)red.
B)blue.
C)green.
D)yellow.
A)red.
B)blue.
C)green.
D)yellow.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
76
The temperature of hydrogen gas is such that electrons are excited by atomic collisions up to the n = 3 atomic energy levels. Emission lines from which spectral sequences result when electrons return to the ground state?
A)Paschen (IR), Balmer (visible), and Lyman (UV) series
B)Lyman (UV) series only
C)Balmer (visible) and Lyman (UV) series
D)Balmer (visible) series only
A)Paschen (IR), Balmer (visible), and Lyman (UV) series
B)Lyman (UV) series only
C)Balmer (visible) and Lyman (UV) series
D)Balmer (visible) series only

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
77
The strong ultraviolet spectral line emitted by hot hydrogen gas is known as the:
A)21-cm line.
B)Balmer line.
C)Paschen line.
D)Lyman line.
A)21-cm line.
B)Balmer line.
C)Paschen line.
D)Lyman line.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
78
The wavelengths λ of the emission lines from hot hydrogen gas depend on an integer n according to:
A)n.
B)1/n2.
C)1/n.
D)n2.
A)n.
B)1/n2.
C)1/n.
D)n2.

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
79
If the wavelengths of Hα and Hß, the first two lines of the hydrogen Balmer series, are 656.3 nm and 486.2 nm, respectively, what is the wavelength of Hγ, the third line of the Balmer series?
A)486.2 nm
B)1875 nm
C)364.6 nm
D)434.1 nm
A)486.2 nm
B)1875 nm
C)364.6 nm
D)434.1 nm

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck
80
What type of radiation is emitted by hot hydrogen gas when electrons jump from the n = 8 level to the n = 7 level of the atoms?
A)52,489 m, in the radio
B)1.905 μm, in the near infrared
C)19.05 μm, in the infrared
D)38.9 nm, in the ultraviolet
A)52,489 m, in the radio
B)1.905 μm, in the near infrared
C)19.05 μm, in the infrared
D)38.9 nm, in the ultraviolet

Unlock Deck
Unlock for access to all 91 flashcards in this deck.
Unlock Deck
k this deck


