Deck 9: Reaction Kinetics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/50
Play
Full screen (f)
Deck 9: Reaction Kinetics
1
For the following reaction H2 (g) + I2 (g) 2 HI (g), the initial rate of formation of hydrogen iodide was found to be 3.2 × 10-3 mol dm-3 s-1. What is the initial rate of consumption of H2?
A) 3.2 × 10-3 mol dm-3 s-1.
B) 1.6 × 10-3 mol dm-3 s-1.
C) 6.4 × 10-3 mol dm-3 s-1.
D) Not possible to determine.
A) 3.2 × 10-3 mol dm-3 s-1.
B) 1.6 × 10-3 mol dm-3 s-1.
C) 6.4 × 10-3 mol dm-3 s-1.
D) Not possible to determine.
B
2
For the following reaction: 2 N2O5 (g) 4 NO2 (g) + O2 (g) the initial rate of formation of O2 was found to be 1.25 × 10-4 mol dm-3 s-1. What is the initial rate of consumption of N2O5?
A) 2.50 × 10-4 mol dm-3 s-1.
B) 1.25 × 10-4 mol dm-3 s-1.
C) 3.75 × 10-4 mol dm-3 s-1.
D) 5.00 × 10-4 mol dm-3 s-1.
A) 2.50 × 10-4 mol dm-3 s-1.
B) 1.25 × 10-4 mol dm-3 s-1.
C) 3.75 × 10-4 mol dm-3 s-1.
D) 5.00 × 10-4 mol dm-3 s-1.
A
3
For the following reaction: BrO3- (aq) + 5 Br- (aq) + 6 H3O+ (aq) 3 Br2 (aq) + 9 H2O (aq), the initial rate of formation of Br2 was found to be 2.4 × 10-3 mol dm-3 s-1. What is the initial rate of formation of H2O?
A) 2.4 × 10-3 mol dm-3 s-1.
B) 2.16 × 10-2 mol dm-3 s-1
C) 4.8 × 10-3 mol dm-3 s-1.
D) 7.2 × 10-3 mol dm-3 s-1.
A) 2.4 × 10-3 mol dm-3 s-1.
B) 2.16 × 10-2 mol dm-3 s-1
C) 4.8 × 10-3 mol dm-3 s-1.
D) 7.2 × 10-3 mol dm-3 s-1.
D
4
For the following reaction: 2 NH3 (g) N2 (g) + 3 H2 (g), the rate of consumption of ammonia was measured as 2 mmol dm-3 s-1; what was the rate of formation of H2?
A) 2 mmol dm-3 s-1.
B) 3 mmol dm-3 s-1.
C) 1 mmol dm-3 s-1.
D) 6 mmol dm-3 s-1.
A) 2 mmol dm-3 s-1.
B) 3 mmol dm-3 s-1.
C) 1 mmol dm-3 s-1.
D) 6 mmol dm-3 s-1.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following are elementary reactions? Select all that apply.
A) H +Cl2 HCl + Cl
B) 2 NO (g) + O2 (g) 2NO2 (g)
C) BrO3- (aq) + 5Br- (aq) + 6H3O+ (aq) 3Br2 (aq) + 9H2O (aq)
D) (CH3)3C+ + H2O [(CH3)3COH2]+
A) H +Cl2 HCl + Cl
B) 2 NO (g) + O2 (g) 2NO2 (g)
C) BrO3- (aq) + 5Br- (aq) + 6H3O+ (aq) 3Br2 (aq) + 9H2O (aq)
D) (CH3)3C+ + H2O [(CH3)3COH2]+

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
6
For a general reaction aA + bB products, the rate equation takes the form: rate of reaction = k[A]a[B]b. Match each of the following with its short description.
-overall order of the reaction = (a + b)
A) (a + b)
B) a
C) b
D) k
-overall order of the reaction = (a + b)
A) (a + b)
B) a
C) b
D) k

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
7
For a general reaction aA + bB products, the rate equation takes the form: rate of reaction = k[A]a[B]b. Match each of the following with its short description.
-order of the reaction with respect to A = a
A) (a + b)
B) a
C) b
D) k
-order of the reaction with respect to A = a
A) (a + b)
B) a
C) b
D) k

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
8
For a general reaction aA + bB products, the rate equation takes the form: rate of reaction = k[A]a[B]b. Match each of the following with its short description.
-order of the reaction with respect to B = b
A) (a + b)
B) a
C) b
D) k
-order of the reaction with respect to B = b
A) (a + b)
B) a
C) b
D) k

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
9
For a general reaction aA + bB products, the rate equation takes the form: rate of reaction = k[A]a[B]b. Match each of the following with its short description.
-rate constant = k
A) (a + b)
B) a
C) b
D) k
-rate constant = k
A) (a + b)
B) a
C) b
D) k

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
10
For a general reaction 2A + 3B products, the rate equation takes the form: rate of reaction = k [A]2[B]1. Select all that apply.
A) The overall order of reaction = 2.
B) The order of reaction with respect to A = 2.
C) The order of the reaction with respect to B = 1.
D) The units of the rate constant k = s-1.
A) The overall order of reaction = 2.
B) The order of reaction with respect to A = 2.
C) The order of the reaction with respect to B = 1.
D) The units of the rate constant k = s-1.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
11
For the general reaction 2A + B products, the rate equation takes the following form: rate of reaction = k[A]2[B]1. What are the units of the rate constant, k?
A) s-1.
B) dm3 mol-1 s-1.
C) mol dm-3 s-1.
D) dm6 mol-2 s-1.
A) s-1.
B) dm3 mol-1 s-1.
C) mol dm-3 s-1.
D) dm6 mol-2 s-1.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
12
The rate of reaction in the following reaction: 2I (g) + Ar (g) I2 (g) + Ar (g) can be defined by which of the following expressions:
A)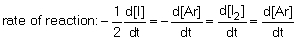 .
.
B)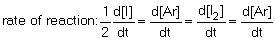 .
.
C)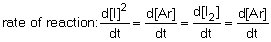 .
.
D)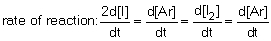 .
.
A)
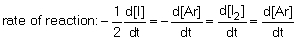 .
.B)
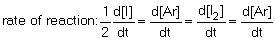 .
.C)
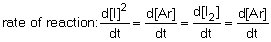 .
.D)
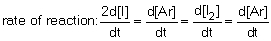 .
.
Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
13
The expression for the rate of reaction for the following elementary reaction NO + O3 → NO2 + O2 is:
A) rate of reaction = k [NO] [O3].
B) rate of reaction = k [NO] [O3]3.
C) rate of reaction = k [NO2] [O2].
D) rate of reaction = .
![<strong>The expression for the rate of reaction for the following elementary reaction NO + O<sub>3</sub> → NO<sub>2</sub> + O<sub>2</sub> is:</strong> A) rate of reaction = k [NO] [O<sub>3</sub>]. B) rate of reaction = k [NO] [O<sub>3</sub>]<sup>3</sup>. C) rate of reaction = k [NO<sub>2</sub>] [O<sub>2</sub>]. D) rate of reaction = .](https://storage.examlex.com/TBO1061/11edadeb_2d74_26fd_a31a_7537929549ad_TBO1061_00.jpg)
A) rate of reaction = k [NO] [O3].
B) rate of reaction = k [NO] [O3]3.
C) rate of reaction = k [NO2] [O2].
D) rate of reaction = .
![<strong>The expression for the rate of reaction for the following elementary reaction NO + O<sub>3</sub> → NO<sub>2</sub> + O<sub>2</sub> is:</strong> A) rate of reaction = k [NO] [O<sub>3</sub>]. B) rate of reaction = k [NO] [O<sub>3</sub>]<sup>3</sup>. C) rate of reaction = k [NO<sub>2</sub>] [O<sub>2</sub>]. D) rate of reaction = .](https://storage.examlex.com/TBO1061/11edadeb_2d74_26fd_a31a_7537929549ad_TBO1061_00.jpg)

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following expressions describes the rate of reaction for the elementary unimolecular process: O3 → O2 + O ?
A) Rate of reaction = k [O2] [O].
B) Rate of reaction = k [O3].
C) Rate of reaction = k [O3]3 .
D) Rate of reaction = k [O2]2 [O].
A) Rate of reaction = k [O2] [O].
B) Rate of reaction = k [O3].
C) Rate of reaction = k [O3]3 .
D) Rate of reaction = k [O2]2 [O].

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
15
For the following elementary reaction: HF + NH3 → F- + NH4+. The rate of consumption of the reactants and rate of formation of products is given by which set of expressions?
A)
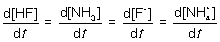
B)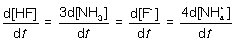
C)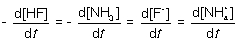
D)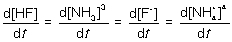
A)
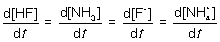
B)
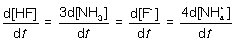
C)
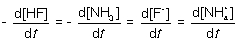
D)
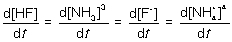

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
16
For the following elementary reaction: 2Br● → Br2. The rate of consumption of the reactant and rate of formation of product is given by which set of expressions?
A)
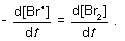
B)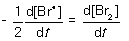
C)
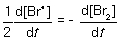
D)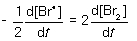
A)
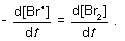
B)
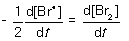
C)
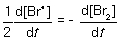
D)
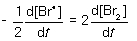

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
17
For the following gas-phase bimolecular elementary reaction 2Cl● → Cl2, which rate equation, expressed in terms of a differential, shows the relationship between the rate of consumption of reactant and rate of reaction?
A)
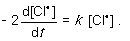
B)
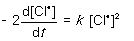
C)
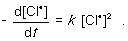
D)
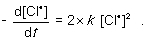
A)
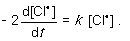
B)
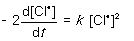
C)
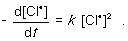
D)
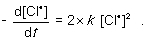

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
18
2 N2O5 (g) 4 NO2 (g) + O2 (g) follows first order reaction kinetics, where the rate of reaction = k [N2O5]. The integrated form of the rate equation is:
A) [N2O5]t = [N2O5]0 - kt.
B) ln[N2O5]t = ln[N2O5]0 - kt .
C) 2[N2O5]t = [N2O5]0 - kt .
D) 2ln[N2O5]t = ln[N2O5]0 - kt .
A) [N2O5]t = [N2O5]0 - kt.
B) ln[N2O5]t = ln[N2O5]0 - kt .
C) 2[N2O5]t = [N2O5]0 - kt .
D) 2ln[N2O5]t = ln[N2O5]0 - kt .

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
19
For the reaction 2 N2O5 (g) 4 NO2 (g) + O2 (g), the rate constant, k = 3.38 × 10-5 s-1. What is the overall reaction order?
A) Zero.
B) First.
C) Second.
D) Third.
A) Zero.
B) First.
C) Second.
D) Third.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
20
For a gas-phase reaction at 308 K, the rate constant k = 0.05 dm3 mol-1 s-1. What is the overall reaction order of the reaction?
A) Zero.
B) First.
C) Second.
D) Third.
A) Zero.
B) First.
C) Second.
D) Third.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
21
For a first order elementary reaction A products, the rate of reaction = k[A]. Select all that apply.
A) .
![<strong>For a first order elementary reaction A <font face=symbol></font> products, the rate of reaction = k[A]. Select all that apply.</strong> A) . B) . C) ln[A]<sub>t</sub> = ln[A]<sub>0</sub> - kt . D) A plot of [A] versus time, t, will give a straight-line graph.](https://storage.examlex.com/TBO1061/11edadeb_2d74_752a_a31a_89e5ce052137_TBO1061_00.jpg)
B) .
![<strong>For a first order elementary reaction A <font face=symbol></font> products, the rate of reaction = k[A]. Select all that apply.</strong> A) . B) . C) ln[A]<sub>t</sub> = ln[A]<sub>0</sub> - kt . D) A plot of [A] versus time, t, will give a straight-line graph.](https://storage.examlex.com/TBO1061/11edadeb_2d74_752b_a31a_0bb606dcf7c0_TBO1061_00.jpg)
C) ln[A]t = ln[A]0 - kt .
D) A plot of [A] versus time, t, will give a straight-line graph.
A) .
![<strong>For a first order elementary reaction A <font face=symbol></font> products, the rate of reaction = k[A]. Select all that apply.</strong> A) . B) . C) ln[A]<sub>t</sub> = ln[A]<sub>0</sub> - kt . D) A plot of [A] versus time, t, will give a straight-line graph.](https://storage.examlex.com/TBO1061/11edadeb_2d74_752a_a31a_89e5ce052137_TBO1061_00.jpg)
B) .
![<strong>For a first order elementary reaction A <font face=symbol></font> products, the rate of reaction = k[A]. Select all that apply.</strong> A) . B) . C) ln[A]<sub>t</sub> = ln[A]<sub>0</sub> - kt . D) A plot of [A] versus time, t, will give a straight-line graph.](https://storage.examlex.com/TBO1061/11edadeb_2d74_752b_a31a_0bb606dcf7c0_TBO1061_00.jpg)
C) ln[A]t = ln[A]0 - kt .
D) A plot of [A] versus time, t, will give a straight-line graph.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
22
A second order elementary reaction is characterised by a half life ( 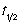 ) that gets shorter with the progress of the reaction.
) that gets shorter with the progress of the reaction.
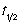 ) that gets shorter with the progress of the reaction.
) that gets shorter with the progress of the reaction.
Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
23
The first order gas-phase process: C2H6 → 2 CH3 has a rate constant, k = 5.36 × 10-4 s-1 at 700 °C. If in a reaction vessel, the initial concentration of ethane was 0.05 mol dm-3, what is its concentration after 250 s?
A) - 0.084 mol dm-3
B) 0.034 mol dm-3
C) 0.044 mol dm-3
D) -3.13 mol dm-3
A) - 0.084 mol dm-3
B) 0.034 mol dm-3
C) 0.044 mol dm-3
D) -3.13 mol dm-3

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
24
A first order reaction, with rate constant k = 3.25 × 10-5 s-1, at 200 °C, is followed experimentally over a period of 1 hour. The initial concentration of the reactant was 0.0280 mol dm-3. What was the concentration of the reactant after one hour?
A) 0.0279 mol dm-3
B) 0.0249 mol dm-3
C) 0.0278 mol dm-3
D) -0.089 mol dm-3
A) 0.0279 mol dm-3
B) 0.0249 mol dm-3
C) 0.0278 mol dm-3
D) -0.089 mol dm-3

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
25
A researcher follows the progress of a reaction and tabulates the concentration of the reactant with respect to time of the reaction. How could the scientist prove that the reaction follows first order kinetics and find the rate constant, k? Select all that apply.
A) Impossible to do.
B) Plot a graph of [reactant] against time, and find a constant half-life (![<strong>A researcher follows the progress of a reaction and tabulates the concentration of the reactant with respect to time of the reaction. How could the scientist prove that the reaction follows first order kinetics and find the rate constant, k? Select all that apply.</strong> A) Impossible to do. B) Plot a graph of [reactant] against time, and find a constant half-life ( ). C) Plot a graph of ln[reactant] against time and get a linear plot. D) Plot a graph of 1/[reactant] against time and get a linear plot.](https://storage.examlex.com/TBO1061/11edadeb_2d74_9c3d_a31a_e987a4603423_TBO1061_00.jpg) ).
).
C) Plot a graph of ln[reactant] against time and get a linear plot.
D) Plot a graph of 1/[reactant] against time and get a linear plot.
A) Impossible to do.
B) Plot a graph of [reactant] against time, and find a constant half-life (
![<strong>A researcher follows the progress of a reaction and tabulates the concentration of the reactant with respect to time of the reaction. How could the scientist prove that the reaction follows first order kinetics and find the rate constant, k? Select all that apply.</strong> A) Impossible to do. B) Plot a graph of [reactant] against time, and find a constant half-life ( ). C) Plot a graph of ln[reactant] against time and get a linear plot. D) Plot a graph of 1/[reactant] against time and get a linear plot.](https://storage.examlex.com/TBO1061/11edadeb_2d74_9c3d_a31a_e987a4603423_TBO1061_00.jpg) ).
).C) Plot a graph of ln[reactant] against time and get a linear plot.
D) Plot a graph of 1/[reactant] against time and get a linear plot.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
26
A researcher follows the progress of a reaction using spectroscopic methods. The half-life, ( 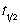 ), is found to depend on the initial concentration of the reactant. This evidence suggests that the reaction is following first order kinetics.
), is found to depend on the initial concentration of the reactant. This evidence suggests that the reaction is following first order kinetics.
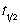 ), is found to depend on the initial concentration of the reactant. This evidence suggests that the reaction is following first order kinetics.
), is found to depend on the initial concentration of the reactant. This evidence suggests that the reaction is following first order kinetics.
Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
27
For a first order reaction, the half-life, 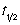 = 237 s. What is the rate constant, k, of the reaction?
= 237 s. What is the rate constant, k, of the reaction?
A) It is not possible to determine the rate constant.
B) 0.0029 s-1.
C) 0.0084 s-1.
D) 342 s.
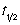 = 237 s. What is the rate constant, k, of the reaction?
= 237 s. What is the rate constant, k, of the reaction?A) It is not possible to determine the rate constant.
B) 0.0029 s-1.
C) 0.0084 s-1.
D) 342 s.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
28
A first order rate constant, k = 3.75 × 10-2 s-1. What is the reaction's half-life, 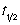 ?
?
A) 18.48 s.
B) 53.33 s.
C) 26.67 s.
D) 0.01875 s-1.
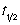 ?
?A) 18.48 s.
B) 53.33 s.
C) 26.67 s.
D) 0.01875 s-1.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
29
A reaction has a rate constant, k = 2.55 × 10-3 mol-1 dm3 s-1. Select all that apply.
A) It is a second order reaction.
B) Double the concentration of the reactant, will quadruple the reaction rate.
C) Its half life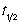 = 272 s.
= 272 s.
D) Its concentration will decay faster than a first order reaction with the same initial rate.
A) It is a second order reaction.
B) Double the concentration of the reactant, will quadruple the reaction rate.
C) Its half life
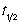 = 272 s.
= 272 s.D) Its concentration will decay faster than a first order reaction with the same initial rate.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
30
Rate of reaction = [A]2[B]
What information does this give us? Select all that apply.
A) The overall reaction order is 2.
B) The reactant order with respect to reactant A is 2.
C) If the concentration of reactant B only is doubled, the rate of reaction quadruples.
D) The overall reaction order is 3.
What information does this give us? Select all that apply.
A) The overall reaction order is 2.
B) The reactant order with respect to reactant A is 2.
C) If the concentration of reactant B only is doubled, the rate of reaction quadruples.
D) The overall reaction order is 3.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
31
If the rate of reaction = [A]2[B], doubling the concentration of [A] will quadruple the rate of reaction. By doubling the concentration of [A] and the concentration of [B] the rate of reaction will increase 16 fold.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
32
Given the data below, which of the following statements is true?
 Select all that apply.
Select all that apply.
A) The reaction is first order.
B) The reaction is second order.
C) The concentration after 700 seconds will be 0.95 × 10-2 mol dm-3.
D) The half-life is independent of temperature.
 Select all that apply.
Select all that apply.A) The reaction is first order.
B) The reaction is second order.
C) The concentration after 700 seconds will be 0.95 × 10-2 mol dm-3.
D) The half-life is independent of temperature.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
33
The rate of reaction depends on the concentration of reactant A only. Calculate the order of the reaction from the data below.

A) Zero order.
B) First order.
C) Second order.
D) Third order.

A) Zero order.
B) First order.
C) Second order.
D) Third order.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
34
Match the following brief description with the term used to describe a reaction mechanism and its reaction steps.
-The step in a reaction mechanism that determines the rate of reaction
A) rate-determining step
B) bimolecular
C) steady state approximation
D) pre-equilibrium
-The step in a reaction mechanism that determines the rate of reaction
A) rate-determining step
B) bimolecular
C) steady state approximation
D) pre-equilibrium

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
35
Match the following brief description with the term used to describe a reaction mechanism and its reaction steps.
-A reaction step that involves two molecules
A) rate-determining step
B) bimolecular
C) steady state approximation
D) pre-equilibrium
-A reaction step that involves two molecules
A) rate-determining step
B) bimolecular
C) steady state approximation
D) pre-equilibrium

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
36
Match the following brief description with the term used to describe a reaction mechanism and its reaction steps.
-The rate of change of concentration of a reactive species, in a sequence of reactions is approximately zero.
A) rate-determining step
B) bimolecular
C) steady state approximation
D) pre-equilibrium
-The rate of change of concentration of a reactive species, in a sequence of reactions is approximately zero.
A) rate-determining step
B) bimolecular
C) steady state approximation
D) pre-equilibrium

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
37
Match the following brief description with the term used to describe a reaction mechanism and its reaction steps.
-A reversible step preceding the rate-determining step
A) rate-determining step
B) bimolecular
C) steady state approximation
D) pre-equilibrium
-A reversible step preceding the rate-determining step
A) rate-determining step
B) bimolecular
C) steady state approximation
D) pre-equilibrium

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
38
In the following reaction sequence the first step is reversible. 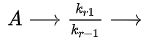
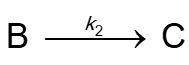 and k2 is small relative to k1 and k-1. Select all that apply.
and k2 is small relative to k1 and k-1. Select all that apply.
A)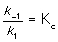 where Kc is the equilibrium constant.
where Kc is the equilibrium constant.
B) The rate-determining step is step two: .
.
C)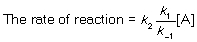 .
.
D)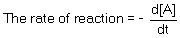 .
.
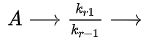
 and k2 is small relative to k1 and k-1. Select all that apply.
and k2 is small relative to k1 and k-1. Select all that apply.A)
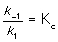 where Kc is the equilibrium constant.
where Kc is the equilibrium constant.B) The rate-determining step is step two:
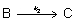 .
.C)
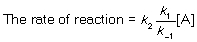 .
.D)
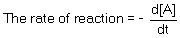 .
.
Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
39
The Arrhenius equation describes how the rate of reaction of many chemical reactions varies with temperature 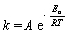 . Match the symbol with its description.
. Match the symbol with its description.
-k
A) rate constant
B) activation energy
C) pre-exponential factor
D) ideal gas constant
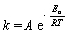 . Match the symbol with its description.
. Match the symbol with its description.-k
A) rate constant
B) activation energy
C) pre-exponential factor
D) ideal gas constant

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
40
The Arrhenius equation describes how the rate of reaction of many chemical reactions varies with temperature 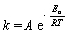 . Match the symbol with its description.
. Match the symbol with its description.
-Ea
A) rate constant
B) activation energy
C) pre-exponential factor
D) ideal gas constant
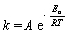 . Match the symbol with its description.
. Match the symbol with its description.-Ea
A) rate constant
B) activation energy
C) pre-exponential factor
D) ideal gas constant

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
41
The Arrhenius equation describes how the rate of reaction of many chemical reactions varies with temperature 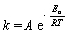 . Match the symbol with its description.
. Match the symbol with its description.
-A
A) rate constant
B) activation energy
C) pre-exponential factor
D) ideal gas constant
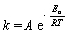 . Match the symbol with its description.
. Match the symbol with its description.-A
A) rate constant
B) activation energy
C) pre-exponential factor
D) ideal gas constant

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
42
The Arrhenius equation describes how the rate of reaction of many chemical reactions varies with temperature 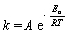 . Match the symbol with its description.
. Match the symbol with its description.
-R
A) rate constant
B) activation energy
C) pre-exponential factor
D) ideal gas constant
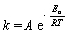 . Match the symbol with its description.
. Match the symbol with its description.-R
A) rate constant
B) activation energy
C) pre-exponential factor
D) ideal gas constant

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
43
A plot of ln k against 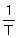 has a slope of - 3257 and an intercept of 25. What is the activation energy (Ea) (kJ mol-1) of the reaction?
has a slope of - 3257 and an intercept of 25. What is the activation energy (Ea) (kJ mol-1) of the reaction?
A) - 3257.
B) 27.
C) 25.
D) 392.
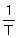 has a slope of - 3257 and an intercept of 25. What is the activation energy (Ea) (kJ mol-1) of the reaction?
has a slope of - 3257 and an intercept of 25. What is the activation energy (Ea) (kJ mol-1) of the reaction?A) - 3257.
B) 27.
C) 25.
D) 392.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
44
A plot of ln (k / s-1) against 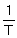 has a slope of - 2575 and an intercept of 31.5. What is the pre-exponential factor A (s-1) of this first order reaction?
has a slope of - 2575 and an intercept of 31.5. What is the pre-exponential factor A (s-1) of this first order reaction?
A) 31.5.
B) - 2575.
C) 21409.
D) 4.8 × 1013,
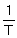 has a slope of - 2575 and an intercept of 31.5. What is the pre-exponential factor A (s-1) of this first order reaction?
has a slope of - 2575 and an intercept of 31.5. What is the pre-exponential factor A (s-1) of this first order reaction?A) 31.5.
B) - 2575.
C) 21409.
D) 4.8 × 1013,

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
45
A plot of ln k against 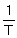 has a slope of - 2752 and an intercept of 30. What is the activation energy (Ea) (kJ mol-1) of the reaction?
has a slope of - 2752 and an intercept of 30. What is the activation energy (Ea) (kJ mol-1) of the reaction?
A) 30.
B) 2752.
C) 23.
D) 331.
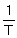 has a slope of - 2752 and an intercept of 30. What is the activation energy (Ea) (kJ mol-1) of the reaction?
has a slope of - 2752 and an intercept of 30. What is the activation energy (Ea) (kJ mol-1) of the reaction?A) 30.
B) 2752.
C) 23.
D) 331.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
46
The activation energy (Ea) of a first order reaction is 70 kJ mol-1. The rate constant (k1) at 25 °C is 2.25 × 10-5 s-1. What is the rate constant (k2) (s-1) at 70 °C?
A) 9.13 × 10-4.
B) 2.39 × 1089.
C) 2.26 × 10-5.
D) 2.25 × 10-5.
A) 9.13 × 10-4.
B) 2.39 × 1089.
C) 2.26 × 10-5.
D) 2.25 × 10-5.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
47
The activation energy (Ea) of a first order reaction is 45 kJ mol-1. The rate constant (k) at 75 °C is 5.75 × 10-4 s-1. What is the rate constant (s-1) at 25 °C?
A) 4.24 × 10-5.
B) 5.74 × 10-4.
C) 1.19 × 10-66.
D) 5.75 × 10-4.
A) 4.24 × 10-5.
B) 5.74 × 10-4.
C) 1.19 × 10-66.
D) 5.75 × 10-4.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
48
At 30 °C and 85 °C the rate constants of a reaction are 3.75 × 10-5 and 2.72 × 10-3 s-1 respectively. What is the activation energy Ea (kJ mol-1)?
A) 1.65.
B) - 70.31.
C) 70.31.
D) 1.02.
A) 1.65.
B) - 70.31.
C) 70.31.
D) 1.02.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
49
The activation energy Ea, of a reaction is - 5 kJ mol-1; this suggests that temperature has no effect on the rate constant k, of the reaction.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following statements are true with respect to the function of a catalyst in a reaction? Select all that apply.
A) It increases the rate of reaction.
B) It changes the amount of product formed.
C) It is chemically changed by the reaction.
D) It must be in the same physical state as the reaction in order to function.
A) It increases the rate of reaction.
B) It changes the amount of product formed.
C) It is chemically changed by the reaction.
D) It must be in the same physical state as the reaction in order to function.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck


