Deck 4: Diatomic Molecules
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/27
Play
Full screen (f)
Deck 4: Diatomic Molecules
1
How do the bond enthalpies and bond lengths for the homonuclear diatomics, H2, Li2, Na2 and K2 change as the group is descended?
A) Bond lengths increase and bond enthalpies increase.
B) Bond lengths increase and bond enthalpies decrease.
C) Bond lengths decrease and bond enthalpies increase.
D) Bond lengths decrease and bond enthalpies decrease.
A) Bond lengths increase and bond enthalpies increase.
B) Bond lengths increase and bond enthalpies decrease.
C) Bond lengths decrease and bond enthalpies increase.
D) Bond lengths decrease and bond enthalpies decrease.
B
2
For a homonuclear diatomic, the bond length is _____the distance of the atomic radius.
Twice
3
In the figure below, the shape of the curve is dictated by which of the following factors? Please select all that apply.
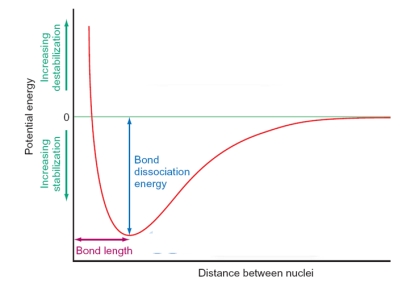
A) At long distances there is no interaction between the atoms as the orbitals are not able to overlap.
B) At very short distances the atoms repel strongly as the positive nuclei come into close contact.
C) The equilibrium between repulsive and attractive forces generates the bond length between the atoms.
D) As the atoms become close enough together the orbitals overlap and the atoms are drawn together as bonding occurs.

A) At long distances there is no interaction between the atoms as the orbitals are not able to overlap.
B) At very short distances the atoms repel strongly as the positive nuclei come into close contact.
C) The equilibrium between repulsive and attractive forces generates the bond length between the atoms.
D) As the atoms become close enough together the orbitals overlap and the atoms are drawn together as bonding occurs.
A, B, C, D
4
The Lewis model can be used to explain the differences in bond strength of F2, O2 and N2.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
5
According to Lewis theory, a nitrogen atom in N2 must share _ electrons to form a stable configuration.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
6
The dot and cross diagram for ammonia (NH3) predicts that the shape at nitrogen is linear.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
7
The bonding of which of the following molecules can be explained by the Lewis model? Please select all that apply.
A) F2
B) NO
C) PF5
D) HF
A) F2
B) NO
C) PF5
D) HF

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
8
____________ species contain unpaired electrons and are affected by an external magnetic field.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
9
The electronegativity of an s or p block species is a constant irrespective of its oxidation state.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
10
Interhalogen compounds of the form XF exist where X is Cl, Br and I. The contribution from X+ F- resonance form is more important than the contribution from the X- F+ resonance form.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
11
Valence bond theory can be used to explain the trigonal planar structure of BF3.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
12
It is possible for an electron in a molecular orbital to be found outside of the boundary created by the combined atomic orbitals.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
13
A σ (sigma) orbital has __________ symmetry about the internuclear axis.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
14
Match the bond order of the dihydrogen species with the appropriate species.
-H2
A) 1
B) 0.5
C) 0
-H2
A) 1
B) 0.5
C) 0

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
15
Match the bond order of the dihydrogen species with the appropriate species.
-H2-
A) 1
B) 0.5
C) 0
-H2-
A) 1
B) 0.5
C) 0

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
16
Match the bond order of the dihydrogen species with the appropriate species.
-H22-
A) 1
B) 0.5
C) 0
-H22-
A) 1
B) 0.5
C) 0

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
17
Taking z as the internuclear axis, how would the orbital formed from the bonding combination of two dz2 orbitals be labelled?
A) σu
B) σg
C) πg
D) πu
A) σu
B) σg
C) πg
D) πu

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
18
The magnetic properties of O2 are very important as they are used to monitor the presence of oxygen in places where oxygen levels are essential. Which of the follow models suggests that dioxygen should be paramagnetic? Please select all that apply.
A) The Lewis model
B) Valence bond theory
C) Molecular orbital theory
D) All of the above
A) The Lewis model
B) Valence bond theory
C) Molecular orbital theory
D) All of the above

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
19
Draw the molecular orbital diagram of OF+ to determine the bond order. OF+ can exist.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
20
MgB2 is a superconducting compound consisting of Mg2+ cations and B22- anions. The diboride anion in this compound is diamagnetic.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following statements are true in relation to homonuclear diatomics? Please select all that apply.
A) Any combination of two atomic orbitals can form a bonding and an antibonding molecular orbital combination.
B) For B2, C2 and N2, the σ bonding combination of the p orbitals are lower in energy than the bonding combination.
C) For O2, the energy level order depends on independent overlap of the s orbitals and p orbitals.
D) For B2, C2 and N2, the energy level order is affected by the mixing of s and p orbitals.
A) Any combination of two atomic orbitals can form a bonding and an antibonding molecular orbital combination.
B) For B2, C2 and N2, the σ bonding combination of the p orbitals are lower in energy than the bonding combination.
C) For O2, the energy level order depends on independent overlap of the s orbitals and p orbitals.
D) For B2, C2 and N2, the energy level order is affected by the mixing of s and p orbitals.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
22
The same general principles apply to constructing molecular orbital diagrams for heteronuclear diatomics as for homonuclear diatomics?

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following statements relating to the molecular orbital energy level diagram of LiH are true? Please select all that apply.
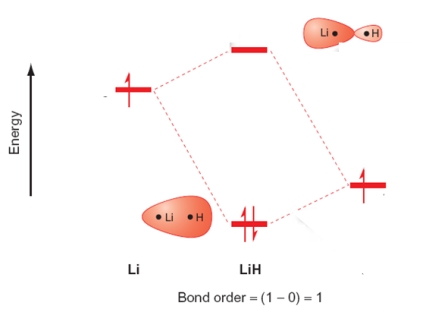
A) Two atomic orbitals, one from each atom, create two molecular orbitals on the LiH molecule.
B) The antibonding combination is labelled 2σu.
C) The 1s orbital of Li and the 1s orbital of H are used to form the molecular orbitals.
D) The out-of-phase combination has a larger contribution from the Li atomic orbital.

A) Two atomic orbitals, one from each atom, create two molecular orbitals on the LiH molecule.
B) The antibonding combination is labelled 2σu.
C) The 1s orbital of Li and the 1s orbital of H are used to form the molecular orbitals.
D) The out-of-phase combination has a larger contribution from the Li atomic orbital.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
24
The wavefunction expression for the antibonding molecular orbital (1σ) in LiH is out of phase = N[H(1s) - Li(2s)] where λ < 1.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
25
In HF, the single bond formed between H and F by the overlap of the 1s on hydrogen with the ___ on F.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
26
The orbital containing electrons and of the highest energy is known as the _____

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following statements relating to the diagram of CO are true? Please select all that apply.
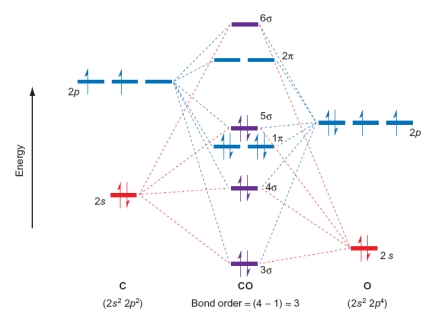
A) Adding electrons to form an anion will lower the bond order.
B) CO is paramagnetic.
C) Adding electrons to form an anion will increase the bond order.
D) CO is diamagnetic.

A) Adding electrons to form an anion will lower the bond order.
B) CO is paramagnetic.
C) Adding electrons to form an anion will increase the bond order.
D) CO is diamagnetic.

Unlock Deck
Unlock for access to all 27 flashcards in this deck.
Unlock Deck
k this deck


