Deck 1: Fundamentals
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/57
Play
Full screen (f)
Deck 1: Fundamentals
1
Systeme International d'Unites is the internationally recognised system of metric units. The system has _____ base units from which all other SI units can be derived.
seven
2
The H-Cl bond length is 127.5 pm. Which of the following lengths is equivalent? Please select all that apply.
A) 1.275 × 10-12 m.
B) 1.275 Å.
C) 0.1275 nm.
D) 1.275 × 10-10 mm.
A) 1.275 × 10-12 m.
B) 1.275 Å.
C) 0.1275 nm.
D) 1.275 × 10-10 mm.
B, C
3
Chemists commonly use the Kelvin (T / K) and Celsius (θ / °C) temperature scales. Match the values in Celsius to the equivalent value on the kelvin temperature scale.
-100 °C
A) 373.15 K
B) 273.15 K
C) 0 K
D) 298 K
-100 °C
A) 373.15 K
B) 273.15 K
C) 0 K
D) 298 K
A
4
Chemists commonly use the Kelvin (T / K) and Celsius (θ / °C) temperature scales. Match the values in Celsius to the equivalent value on the kelvin temperature scale.
-0 °C
A) 373.15 K
B) 273.15 K
C) 0 K
D) 298 K
-0 °C
A) 373.15 K
B) 273.15 K
C) 0 K
D) 298 K

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
5
Chemists commonly use the Kelvin (T / K) and Celsius (θ / °C) temperature scales. Match the values in Celsius to the equivalent value on the kelvin temperature scale.
--273.15 °C
A) 373.15 K
B) 273.15 K
C) 0 K
D) 298 K
--273.15 °C
A) 373.15 K
B) 273.15 K
C) 0 K
D) 298 K

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
6
Chemists commonly use the Kelvin (T / K) and Celsius (θ / °C) temperature scales. Match the values in Celsius to the equivalent value on the kelvin temperature scale.
-25 °C
A) 373.15 K
B) 273.15 K
C) 0 K
D) 298 K
-25 °C
A) 373.15 K
B) 273.15 K
C) 0 K
D) 298 K

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
7
The derived SI unit for pressure is Pa (pascals). What is this in base SI units given that pressure = force/area?
A) atm.
B) Kg m-1 s-2.
C) bar.
D) g mm-1 s-2.
A) atm.
B) Kg m-1 s-2.
C) bar.
D) g mm-1 s-2.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following will the isotopes of an element have in common? Please select all that apply.
A) The same number of protons.
B) The same number of neutrons.
C) A different number of electrons.
D) A different number of neutrons.
A) The same number of protons.
B) The same number of neutrons.
C) A different number of electrons.
D) A different number of neutrons.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
9
Element X has the atomic symbol 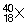 . How many neutrons are there in the nucleus?
. How many neutrons are there in the nucleus?
A) 18.
B) 36.
C) 22.
D) 40.
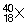 . How many neutrons are there in the nucleus?
. How many neutrons are there in the nucleus?A) 18.
B) 36.
C) 22.
D) 40.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
10
Isotopes of an element chemically react in the same manner but differ in their physical properties.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
11
The number of atoms of xenon in 1 mole is equal to _________ constant.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
12
What mass (in g) of gold contains 8.9 mol of Au?
A) 12 g.
B) 0.045 g.
C) 1753.033 g.
D) 6.022 × 1023 g.
A) 12 g.
B) 0.045 g.
C) 1753.033 g.
D) 6.022 × 1023 g.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
13
How many moles of molecules are contained in 232 g of SiO2?
A) 0.26 mol.
B) 3.86 mol.
C) 13939.49 mol.
D) 5.26 mol.
A) 0.26 mol.
B) 3.86 mol.
C) 13939.49 mol.
D) 5.26 mol.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
14
An organic compound is found to contain by mass, 76.57% carbon, 6.43% hydrogen and 17% oxygen and a relative molecular mass (Mr) of 94.12 g mol-1. The molecular formula of the compound is ______.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
15
The empirical formula of a substance tells you the actual number of different elements in a molecule.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
16
The combustion of glucose in a plentiful of supply of air is given by the following equation: W C6H12O6 (s) + X O2(g) → Y CO2 (g) + Z H2O (l). What are the values of the stoichiometric coefficients W, X, Y, and Z?
Please select all that apply.
A) W = 2.
B) X = 6.
C) Y = 6.
D) Z = 6.
Please select all that apply.
A) W = 2.
B) X = 6.
C) Y = 6.
D) Z = 6.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
17
The balanced equation for the combustion of sucrose in a plentiful supply of air is: C12H22O11 (s) + 12 O2 (g) → 12 CO2 (g) + 11 H2O (l). What mass of oxygen is needed to react exactly with 500 g of sucrose?
A) 6000 g.
B) 578 g.
C) 384 g.
D) 500 g.
A) 6000 g.
B) 578 g.
C) 384 g.
D) 500 g.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
18
What is the oxidation state of C in Li2CO3?
A) +1.
B) +2.
C) +3.
D) +4.
A) +1.
B) +2.
C) +3.
D) +4.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
19
What is the oxidation state of N in KNO2?
A) +1.
B) +2.
C) +3.
D) +4.
A) +1.
B) +2.
C) +3.
D) +4.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
20
In the reaction of lithium with water lithium has been ________.
2 Li(s) + 2 H2O(l) → 2 LiOH(aq) + H2(g)
2 Li(s) + 2 H2O(l) → 2 LiOH(aq) + H2(g)

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
21
The systematic name for SF6 is sulphur ____fluoride

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
22
Match the following brief description with the correct term:
-involves the transfer of a proton
A) Acid-base reaction
B) Precipitation reaction
C) Redox reaction
D) Complexation reaction
-involves the transfer of a proton
A) Acid-base reaction
B) Precipitation reaction
C) Redox reaction
D) Complexation reaction

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
23
Match the following brief description with the correct term:
-involves the formation of a solid product when two solutions are mixed
A) Acid-base reaction
B) Precipitation reaction
C) Redox reaction
D) Complexation reaction
-involves the formation of a solid product when two solutions are mixed
A) Acid-base reaction
B) Precipitation reaction
C) Redox reaction
D) Complexation reaction

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
24
Match the following brief description with the correct term:
-involves the formation of a complex
A) Acid-base reaction
B) Precipitation reaction
C) Redox reaction
D) Complexation reaction
-involves the formation of a complex
A) Acid-base reaction
B) Precipitation reaction
C) Redox reaction
D) Complexation reaction

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
25
The systematic name for XeF4 is xenon _____fluoride

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
26
The systematic name for SiO2 is silicon __oxide

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
27
In the reaction between zinc and hydrogen chloride, what is the oxidation state change of zinc?
Zn (s) + HCl (l) → ZnCl2 (s) + H2 (g)
A) +2.
B) -2.
C) 0.
D) +1.
Zn (s) + HCl (l) → ZnCl2 (s) + H2 (g)
A) +2.
B) -2.
C) 0.
D) +1.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
28
23.75 g of potassium iodide was dissolved in water and the solution made up to 250 cm3. What is the concentration of the solution in mol dm-3?
A) 0.38
B) 0.57
C) 95
D) 0.095
A) 0.38
B) 0.57
C) 95
D) 0.095

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
29
In order to make a 500 cm3 solution of potassium chloride, concentration 2 mol dm-3, what mass (in g) of potassium chloride must be dissolved in water?
A) 1 g.
B) 74550 g.
C) 149.10 g.
D) 74.55 g.
A) 1 g.
B) 74550 g.
C) 149.10 g.
D) 74.55 g.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
30
When the equivalence point of a titration has been reached, the following statements apply. Please select all that apply.
A) There are equal volumes of reagents.
B) The amount of reagent added from the burette has reacted exactly with the solution in the conical flask.
C) There are equal amounts (mass) of reagents.
D) An indicator added would change colour at this point.
A) There are equal volumes of reagents.
B) The amount of reagent added from the burette has reacted exactly with the solution in the conical flask.
C) There are equal amounts (mass) of reagents.
D) An indicator added would change colour at this point.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
31
In order to make a 750 cm3 solution of potassium iodate (KIO3), with concentration 2.5 mol dm-3, what mass (in g) of potassium iodate must be dissolved?
A) 401 g.
B) 401241 g.
C) 1.875 g.
D) 535 g.
A) 401 g.
B) 401241 g.
C) 1.875 g.
D) 535 g.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
32
A solution of potassium iodate (KIO3) has a concentration of 1.75 mol dm-3. What volume (in dm3) of solution contains exactly 50 g?
A) 1.335 × 10-4 .
B) 0.134
C) 0.0875
D) 2.45
A) 1.335 × 10-4 .
B) 0.134
C) 0.0875
D) 2.45

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
33
In a titration, a standard solution of hydrochloric acid (concentration 0.2 mol dm-3) was added to 25 cm3 of a sodium hydroxide solution, concentration unknown. On addition of 18.5 cm3 HCl, the equivalence point was reached. What is the concentration of the sodium hydroxide solution?
A) 0.2
B) 3.7 × 10-3.
C) 0.296
D) 0.148
A) 0.2
B) 3.7 × 10-3.
C) 0.296
D) 0.148

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
34
A 150 cm3 solution containing Br- was treated with excess AgNO3 in order to find the Br- concentration in the solution. 0.873 g of AgBr was precipitated out. What was the concentration (in mol dm-3) of Br- in the unknown solution?
A) 4.65 × 10-3.
B) 0.031
C) 3.10 × 10-5.
D) 0.073
A) 4.65 × 10-3.
B) 0.031
C) 3.10 × 10-5.
D) 0.073

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
35
A standard solution of Ba(OH)2 (concentration 0.25 mol dm-3) was added to 50 cm3 of a HNO3 solution, concentration unknown. On addition of 28.35 cm3 Ba(OH)2, the equivalence point was reached. What is the concentration (in mol dm-3) of the sodium hydroxide solution?
Ba(OH)2 (aq) + 2 HNO3 (aq) → Ba(NO3)2 (aq) + 2 H2O (l)
A) 0.014
B) 0.28
C) 0.25
D) 7.0875 × 10-3
Ba(OH)2 (aq) + 2 HNO3 (aq) → Ba(NO3)2 (aq) + 2 H2O (l)
A) 0.014
B) 0.28
C) 0.25
D) 7.0875 × 10-3

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
36
An endothermic reaction is one in which the system releases energy and warms the surroundings.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
37
An exothermic reaction has a ________ enthalpy change.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
38
The enthalpy change of a reaction is the heat transferred between the reaction and its surroundings at constant ________.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
39
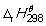 What information does this symbol convey about an enthalpy change? Please select all that apply.
What information does this symbol convey about an enthalpy change? Please select all that apply.A)
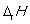 is measured at a pressure of 1 atm.
is measured at a pressure of 1 atm.B)
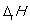 is measured at a temperature of 298 K.
is measured at a temperature of 298 K.C)
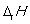 is measured under standard conditions.
is measured under standard conditions.D)
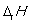 is the standard enthalpy change of formation.
is the standard enthalpy change of formation.
Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
40
The combustion of glucose in oxygen at 298 K is given by the following thermochemical equation:
C6H12O6 (s) + 6O2 → 6CO2 (g) + 6H2O (l)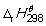 = -2808 kJ mol-1. How much heat energy (in kJ) is supplied when 275 g of glucose burn in a plentiful supply of air so that combustion is complete?
= -2808 kJ mol-1. How much heat energy (in kJ) is supplied when 275 g of glucose burn in a plentiful supply of air so that combustion is complete?
A) 2808.
B) 4286.19
C) 772.20
D) 772200.
C6H12O6 (s) + 6O2 → 6CO2 (g) + 6H2O (l)
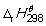 = -2808 kJ mol-1. How much heat energy (in kJ) is supplied when 275 g of glucose burn in a plentiful supply of air so that combustion is complete?
= -2808 kJ mol-1. How much heat energy (in kJ) is supplied when 275 g of glucose burn in a plentiful supply of air so that combustion is complete?A) 2808.
B) 4286.19
C) 772.20
D) 772200.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
41
The combustion of butane in oxygen at 298 K is given by the following thermochemical equation:
2 C4H10 (g) + 13 O2 → 8 CO2 (g) + 10 H2O (l)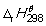 = -2878 kJ mol-1. How much heat energy (in kJ) is supplied when 775 g of butane burn in a plentiful supply of air so that combustion is complete?
= -2878 kJ mol-1. How much heat energy (in kJ) is supplied when 775 g of butane burn in a plentiful supply of air so that combustion is complete?
A) 38370.
B) 2878.
C) 2230.45
D) 2230450.
2 C4H10 (g) + 13 O2 → 8 CO2 (g) + 10 H2O (l)
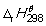 = -2878 kJ mol-1. How much heat energy (in kJ) is supplied when 775 g of butane burn in a plentiful supply of air so that combustion is complete?
= -2878 kJ mol-1. How much heat energy (in kJ) is supplied when 775 g of butane burn in a plentiful supply of air so that combustion is complete?A) 38370.
B) 2878.
C) 2230.45
D) 2230450.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
42
When a chemical bond is broken, energy is required to break the bond. This means the bond breaking process is endothermic.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
43
Match the description with the state of matter it describes.
-A rigid form of matter, it has a shape and occupies a fixed volume (at a particular temperature and pressure).
A) solid
B) gas
C) liquid
-A rigid form of matter, it has a shape and occupies a fixed volume (at a particular temperature and pressure).
A) solid
B) gas
C) liquid

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
44
Match the description with the state of matter it describes.
-A fluid form of matter, it does not have a fixed shape and will expand to fill the container it is in.
A) solid
B) gas
C) liquid
-A fluid form of matter, it does not have a fixed shape and will expand to fill the container it is in.
A) solid
B) gas
C) liquid

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
45
Match the description with the state of matter it describes.
-A fluid form of matter, it occupies a fixed volume (at a particular temperature and pressure) but does not have a fixed shape.
A) solid
B) gas
C) liquid
-A fluid form of matter, it occupies a fixed volume (at a particular temperature and pressure) but does not have a fixed shape.
A) solid
B) gas
C) liquid

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
46
Match the transition between two states of matter with the enthalpy change for the process.
-ΔvapHo
A) Liquid to gas
B) Liquid to solid
C) Gas to solid
D) Solid to liquid
-ΔvapHo
A) Liquid to gas
B) Liquid to solid
C) Gas to solid
D) Solid to liquid

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
47
Match the transition between two states of matter with the enthalpy change for the process.
-ΔfreezingHo
A) Liquid to gas
B) Liquid to solid
C) Gas to solid
D) Solid to liquid
-ΔfreezingHo
A) Liquid to gas
B) Liquid to solid
C) Gas to solid
D) Solid to liquid

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
48
Match the transition between two states of matter with the enthalpy change for the process.
-Δreverse subHo
A) Liquid to gas
B) Liquid to solid
C) Gas to solid
D) Solid to liquid
-Δreverse subHo
A) Liquid to gas
B) Liquid to solid
C) Gas to solid
D) Solid to liquid

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
49
Match the transition between two states of matter with the enthalpy change for the process.
-ΔfusHo
A) Liquid to gas
B) Liquid to solid
C) Gas to solid
D) Solid to liquid
-ΔfusHo
A) Liquid to gas
B) Liquid to solid
C) Gas to solid
D) Solid to liquid

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
50
Match the non-covalent interaction with an example of a molecule pair that the non-covalent interaction acts between.
-London dispersion
A) C2H6, C4H10
B) CHCl3, CH3OH
C) Na+ and H2O
D) CH3OH, H2O
-London dispersion
A) C2H6, C4H10
B) CHCl3, CH3OH
C) Na+ and H2O
D) CH3OH, H2O

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
51
Match the non-covalent interaction with an example of a molecule pair that the non-covalent interaction acts between.
-Dipole-dipole
A) C2H6, C4H10
B) CHCl3, CH3OH
C) Na+ and H2O
D) CH3OH, H2O
-Dipole-dipole
A) C2H6, C4H10
B) CHCl3, CH3OH
C) Na+ and H2O
D) CH3OH, H2O

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
52
Match the non-covalent interaction with an example of a molecule pair that the non-covalent interaction acts between.
-Ion-dipole
A) C2H6, C4H10
B) CHCl3, CH3OH
C) Na+ and H2O
D) CH3OH, H2O
-Ion-dipole
A) C2H6, C4H10
B) CHCl3, CH3OH
C) Na+ and H2O
D) CH3OH, H2O

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
53
Match the non-covalent interaction with an example of a molecule pair that the non-covalent interaction acts between.
-Hydrogen bond
A) C2H6, C4H10
B) CHCl3, CH3OH
C) Na+ and H2O
D) CH3OH, H2O
-Hydrogen bond
A) C2H6, C4H10
B) CHCl3, CH3OH
C) Na+ and H2O
D) CH3OH, H2O

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
54
London dispersion interactions arise because of the instantaneous ______ that can exist due to the uneven electron distribution within a molecule at a particular instant. This induces a dipole in an adjacent molecule.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following will alter the position of equilibrium of a reaction in dynamic equilibrium? Please select all that apply.
A) Pressure of reacting gases.
B) Temperature of reacting solution.
C) Concentration of reacting substances in solution.
D) Time.
A) Pressure of reacting gases.
B) Temperature of reacting solution.
C) Concentration of reacting substances in solution.
D) Time.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
56
Increasing the temperature of an endothermic reaction in dynamic equilibrium will favour the reactants.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
57
H2 (g) + I2 (g) → 2HI (g) Construct an expression for the equilibrium constant Kp in terms of partial pressures of the reactant and product gases.
A)
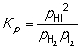
B)
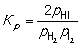
C)
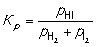
D)
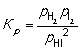
A)
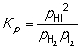
B)
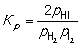
C)
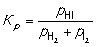
D)
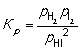

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck


