Deck 7: Cyclic Compounds and Reaction Stereochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/25
Play
Full screen (f)
Deck 7: Cyclic Compounds and Reaction Stereochemistry
1
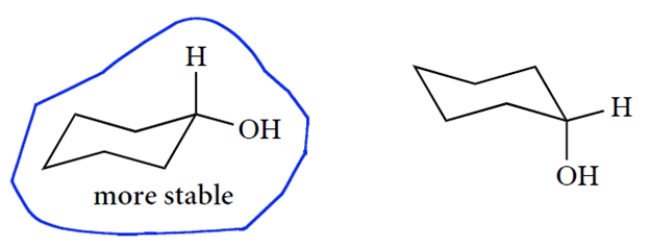
Draw the two chair conformations of cyclohexanol and circle the more stable conformation of the two. You only need to show the carbon-hydrogen bond at the carbon bearing the OH group (†). Bonds must be properly positioned for credit.

The two chair structures are as follows (and there are many correct ways to draw them). The equatorial conformation is more stable.

2
On the template, complete the other chair conformation of cis-1,3-dimethylcyclohexane and circle the more stable conformation.

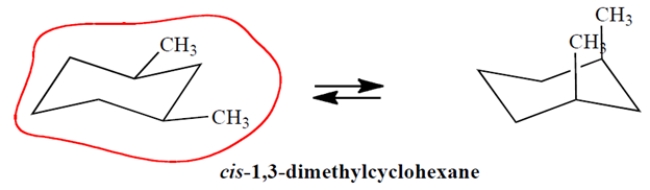
3
Alkaline potassium permanganate (an achiral reagent) adds two -OH groups to a double bond, as shown in the reaction.
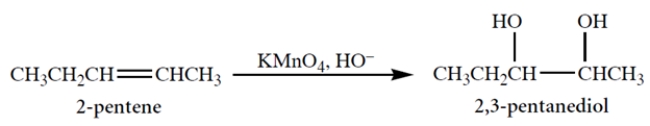 This reaction is known to be a syn-addition. This means that cis-2-pentene will give as product
This reaction is known to be a syn-addition. This means that cis-2-pentene will give as product
A) (2S,3S)-2,3-pentanediol.
B) the racemate of the compound in a.
C) (2R,3S)-2,3-pentanediol.
D) the racemate of the compound in c.
E) meso-2,3-pentanediol.
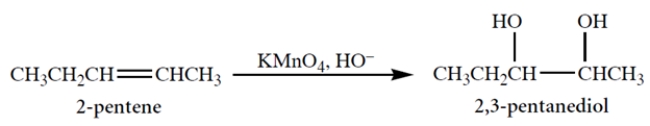 This reaction is known to be a syn-addition. This means that cis-2-pentene will give as product
This reaction is known to be a syn-addition. This means that cis-2-pentene will give as productA) (2S,3S)-2,3-pentanediol.
B) the racemate of the compound in a.
C) (2R,3S)-2,3-pentanediol.
D) the racemate of the compound in c.
E) meso-2,3-pentanediol.
D
4
Complete the structure on the right by adding groups to the "dangling bonds" to give the product of the chair flip.
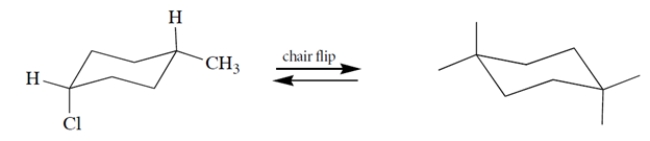


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
5
The two conformations of the hydrocarbon given in planar form at the left are compound ________________ and compound ________________.
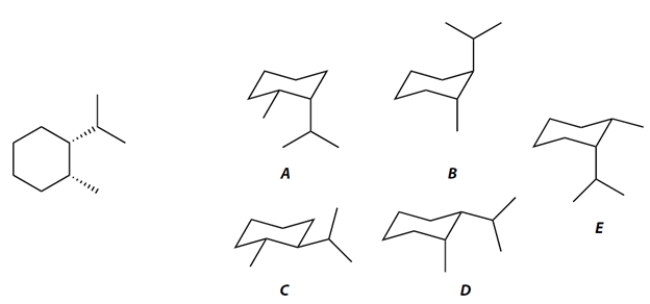 The more stable conformation of these two is compound ________________.
The more stable conformation of these two is compound ________________.
 The more stable conformation of these two is compound ________________.
The more stable conformation of these two is compound ________________.
Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
6
Which one of these structures is a properly drawn chair conformation of trans-1,2-dimethylcyclohexane?
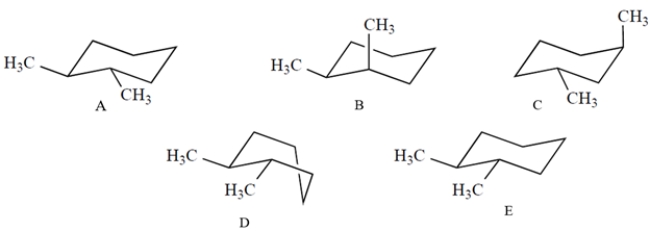
A) compound A
B) compound B
C) compound C
D) compound D
E) compound E

A) compound A
B) compound B
C) compound C
D) compound D
E) compound E

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
7
Which one of these molecules can readily undergo the chair-to-chair interconversion ("chair flip")?
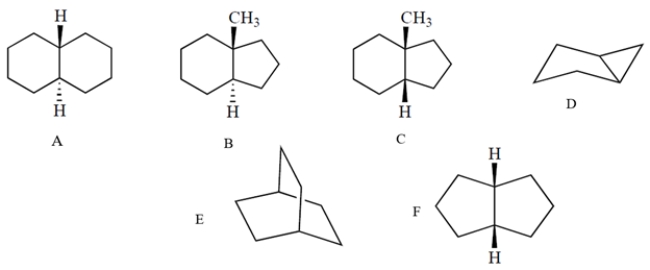
A) compound A
B) compound B
C) compound C
D) compound D
E) compound Ef.
compound F

A) compound A
B) compound B
C) compound C
D) compound D
E) compound Ef.
compound F

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
8
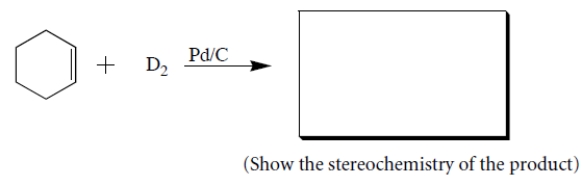
(a) Give the structures of all stereoisomers formed as products in the reaction. Draw only those isomers that are consistent with the known stereospecificity of the reaction! (D = deuterium = the isotope of hydrogen with atomic mass = 2.) (b) Are the stereoisomers you drew formed in the same or different amounts?
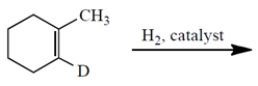
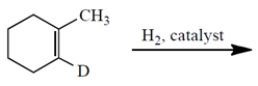

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
9
Fill in the chair template to show the ring flipped conformation of the structure on the left, numbering the carbons in the ring clockwise from the carbon labeled "1" (include hydrogens). Then circle the conformation that is the more stable.
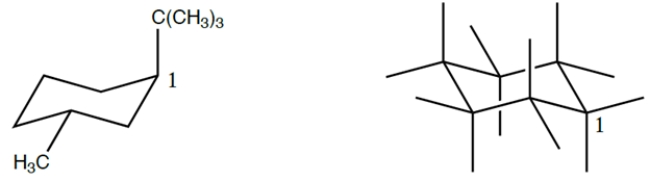
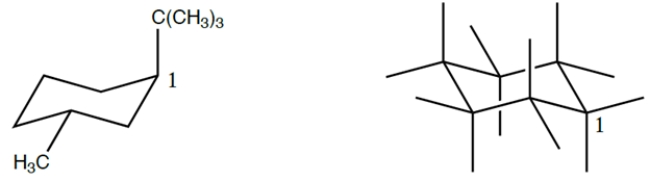

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
10
Examine the chair conformations A-F for the structure on the left.
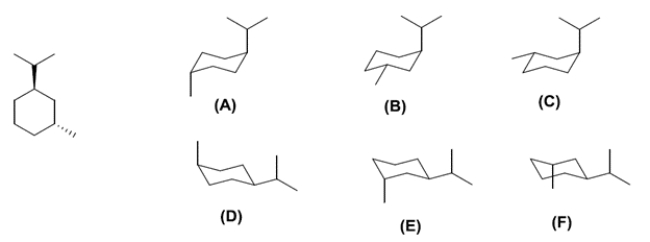 The two correct conformations of the hydrocarbon shown in the line and wedge form are compound ________________ and compound ________________. Of the two compounds you identified, compound ________________ is more stable.
The two correct conformations of the hydrocarbon shown in the line and wedge form are compound ________________ and compound ________________. Of the two compounds you identified, compound ________________ is more stable.
 The two correct conformations of the hydrocarbon shown in the line and wedge form are compound ________________ and compound ________________. Of the two compounds you identified, compound ________________ is more stable.
The two correct conformations of the hydrocarbon shown in the line and wedge form are compound ________________ and compound ________________. Of the two compounds you identified, compound ________________ is more stable.
Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
11
For the structure:
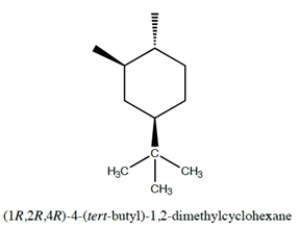 Fill in the bonds and group structures that show the positions of the three functional groups (two methyl groups and a tert-butyl group) using the templates for the chair and the 'flipped' chair conformations provided. You do not need to include hydrogens. Use the ring numbering as indicated.
Fill in the bonds and group structures that show the positions of the three functional groups (two methyl groups and a tert-butyl group) using the templates for the chair and the 'flipped' chair conformations provided. You do not need to include hydrogens. Use the ring numbering as indicated.

 Fill in the bonds and group structures that show the positions of the three functional groups (two methyl groups and a tert-butyl group) using the templates for the chair and the 'flipped' chair conformations provided. You do not need to include hydrogens. Use the ring numbering as indicated.
Fill in the bonds and group structures that show the positions of the three functional groups (two methyl groups and a tert-butyl group) using the templates for the chair and the 'flipped' chair conformations provided. You do not need to include hydrogens. Use the ring numbering as indicated.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
12
Select the bridged bicyclic compound with the formula C8H12 that violates Bredt's rule.
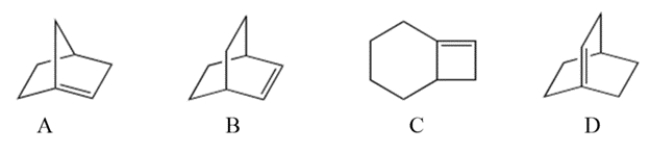
A) compound A
B) compound B
C) compound C
D) compound D

A) compound A
B) compound B
C) compound C
D) compound D

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
13
Draw the most stable chair conformation of each of the following alcohols.
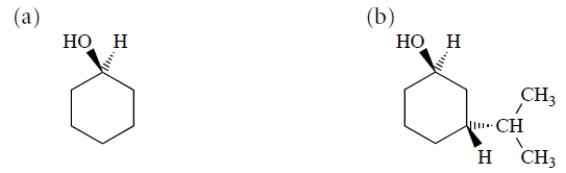
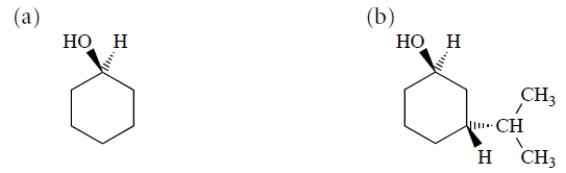

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
14
Consider the following reaction and its stereochemistry.
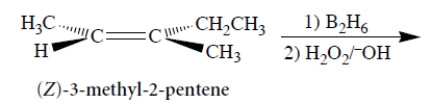 Select the correct statements.
Select the correct statements.
A) The product of this reaction is enantiomerically pure (2R,3S)-3-methyl-2-pentanol.
B) The product of this reaction is equal amounts of (2R,3S)-3-methyl-2-pentanol and (2S,3R)-3-methyl-2-pentanol.
C) The product of this reaction is enantiomerically pure (2S,3R)-3-methyl-2-pentanol.
D) The product of this reaction is considerable but unequal amounts of both (2S,3R)-3-methyl-2-pentanol and (2R,3S)-3-methyl-2-pentanol.
E) The product of the reaction is all four stereoisomers of 3-methyl-2-pentanol in different but substantial amounts.
F) The product of this reaction is enantiomerically pure (2R,3R)-3-methyl-2-pentanol.
G) The product of this reaction is enantiomerically pure (2S,3S)-3-methyl-2-pentanol.
H) The product of this reaction is (2R,3R)-3-methyl-2-pentanol and an equal amount of its enantiomer.
I) The product of this reaction is a racemate.
J) The product of this reaction is meso-3-methyl-2-pentanol.
K) The product of this reaction is racemic 3-methyl-3-pentanol.
L) The E isomer of the starting material would give the diastereomer(s) of the products formed from the Z isomer above.
M) The E isomer of the starting material would give the enantiomer(s) of the products formed from the Z isomer above.
N) The product of this reaction is a secondary alcohol.
O) The product of this reaction is a tertiary alcohol.
 Select the correct statements.
Select the correct statements.A) The product of this reaction is enantiomerically pure (2R,3S)-3-methyl-2-pentanol.
B) The product of this reaction is equal amounts of (2R,3S)-3-methyl-2-pentanol and (2S,3R)-3-methyl-2-pentanol.
C) The product of this reaction is enantiomerically pure (2S,3R)-3-methyl-2-pentanol.
D) The product of this reaction is considerable but unequal amounts of both (2S,3R)-3-methyl-2-pentanol and (2R,3S)-3-methyl-2-pentanol.
E) The product of the reaction is all four stereoisomers of 3-methyl-2-pentanol in different but substantial amounts.
F) The product of this reaction is enantiomerically pure (2R,3R)-3-methyl-2-pentanol.
G) The product of this reaction is enantiomerically pure (2S,3S)-3-methyl-2-pentanol.
H) The product of this reaction is (2R,3R)-3-methyl-2-pentanol and an equal amount of its enantiomer.
I) The product of this reaction is a racemate.
J) The product of this reaction is meso-3-methyl-2-pentanol.
K) The product of this reaction is racemic 3-methyl-3-pentanol.
L) The E isomer of the starting material would give the diastereomer(s) of the products formed from the Z isomer above.
M) The E isomer of the starting material would give the enantiomer(s) of the products formed from the Z isomer above.
N) The product of this reaction is a secondary alcohol.
O) The product of this reaction is a tertiary alcohol.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
15
Complete the reaction by giving the structures of the missing organic products. (Ignore by-products.) (Note that D = deuterium, an isotope of hydrogen, that reacts like hydrogen.)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
16
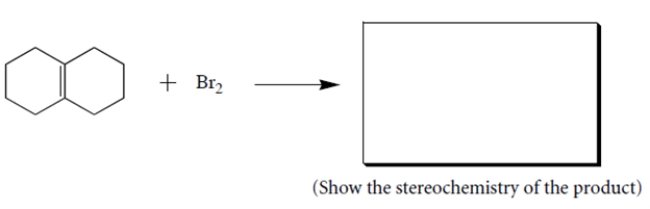
Complete the following reaction by giving the structures of the missing organic products.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
17
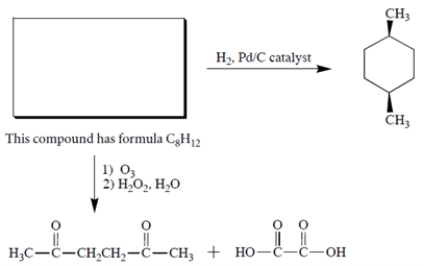
Complete the following reaction by giving the structure of the missing organic starting material.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
18
Cis-1,3-dimethylcyclohexane contains considerably smaller percentage of diaxial conformation than trans-1,2-dimethylcyclohexane. However, cis-cyclohexane-1,3-diol has a higher percentage diaxial conformation than trans-cyclohexane-1,2-diol. (For purposes of this problem, you can assume that an -OH group and a -CH3 group have about the same size.) Explain this difference. A drawing of the diaxial chair conformation of cis-cyclohexane-1,3-diol must be part of your answer.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
19
Which statement best describes the relationship of these two molecules?

A) diastereomers that are meso compounds
B) diastereomers that are not meso compound
C) enantiomers that are meso compounds
D) enantiomers that are not meso compounds
E) identical molecules that are mesof.
identical molecules that are not meso

A) diastereomers that are meso compounds
B) diastereomers that are not meso compound
C) enantiomers that are meso compounds
D) enantiomers that are not meso compounds
E) identical molecules that are mesof.
identical molecules that are not meso

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
20
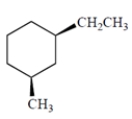
Draw the two chair conformations of the hydrocarbon. Be sure you draw the correct enantiomer! Indicate the more stable of the two conformations.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
21
Consider bromine addition to alkene A below, which is the pure enantiomer. As a pure enantiomer, it is optically active, and measurement of its optical activity shows that it has (+) optical rotation.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
22
Draw the two chair conformations of the compound and circle the more stable conformation. Be sure to draw this compound and not its enantiomer.
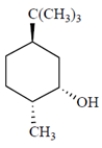


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
23
Olean is the sex attractant of the fruit fly. The female emits this compound to signal her readiness for mating. (This has been used to control fruit-fly populations in orchards.) (The numbers on the structure are for reference in the questions below.)
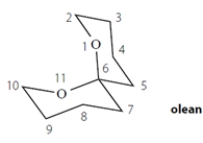 a. Determine whether olean is chiral. Explain how you know. No credit is given without a credible explanation, which should include structures.
a. Determine whether olean is chiral. Explain how you know. No credit is given without a credible explanation, which should include structures.
b. Does olean have any stereocenters?
If you determined that olean has stereocenters, how many does it have? If none, write NONE.
c. Does olean have any asymmetric carbons?
If you determined that olean has asymmetric carbons, how many does it have? If none, write NONE.
 a. Determine whether olean is chiral. Explain how you know. No credit is given without a credible explanation, which should include structures.
a. Determine whether olean is chiral. Explain how you know. No credit is given without a credible explanation, which should include structures.b. Does olean have any stereocenters?
If you determined that olean has stereocenters, how many does it have? If none, write NONE.
c. Does olean have any asymmetric carbons?
If you determined that olean has asymmetric carbons, how many does it have? If none, write NONE.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
24
Limonene is a precursor to the biosynthesis of menthol-a component of peppermint oil. The structure of the levorotatory enantiomer of menthol is given.
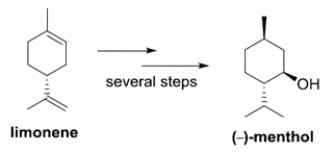 Which structure represents the dextrorotatory enantiomer of menthol?
Which structure represents the dextrorotatory enantiomer of menthol?
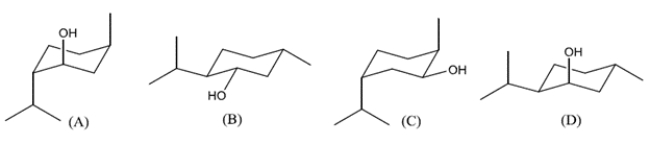
A) compound A
B) compound B
C) compound C
D) compound D
 Which structure represents the dextrorotatory enantiomer of menthol?
Which structure represents the dextrorotatory enantiomer of menthol?
A) compound A
B) compound B
C) compound C
D) compound D

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
25
An achiral alkene A with the molecular formula C8H16 reacts with B2H6, then H2O2/−OH, to give a single alcohol B, C8H18O, that can be resolved into enantiomers. When alkene A is subjected to Hg(OAc)2/H2O, then NaBH4, a single achiral alcohol C is obtained that has different properties from B but has the same formula. Reaction of A with O3, then H2O2/H2O, gives a ketone D (C7H14O) as one product. Catalytic hydrogenation of alkene A yields 3-ethyl-2-methylpentane. Deduce the structures of compounds A, B, C, and D, and be sure each structure is properly labeled.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck


