Deck 2: Alkanes and Organic Nomenclature
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/25
Play
Full screen (f)
Deck 2: Alkanes and Organic Nomenclature
1
Consider the Newman projection. Give the systematic (IUPAC) name of this alkane.
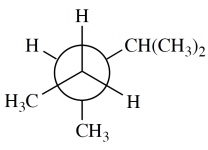

This tests your ability to "decode" a Newman projection. Always remember that the "circle" represents only one carbon of a bond; there is a second carbon obscured behind it that you can't see-the one with the three bonds to the periphery. Hopefully you drew a structure before you constructed the name:
The name then follows: 2,3-dimethylpentane.

The name then follows: 2,3-dimethylpentane.
2
Select all Newman projections that are valid Newman projections for stable conformations of butane. ("Stable" means located at any energy minimum in a plot of energy vs. dihedral angle.)
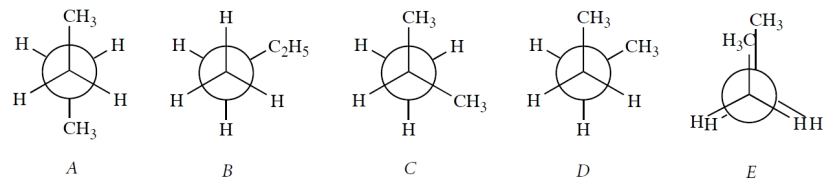

A, B, and D. Projection B is a projection of the C1-C2 bond, whereas the other correct projections are projections of the C2-C3 bond. Projection C is not butane, and projection E is not a stable conformation of butane. Although the gauche conformation D is less stable than the anti conformation A, it lies at an energy minimum and certainly exists.
3
Consider the compound:
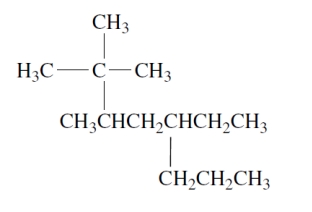 The largest substituent group mentioned (cited) explicitly in the name of this compound is
The largest substituent group mentioned (cited) explicitly in the name of this compound is
A) tert-butyl.
B) methyl.
C) ethyl.
D) propyl.
E) octyl.f.
some other group.
 The largest substituent group mentioned (cited) explicitly in the name of this compound is
The largest substituent group mentioned (cited) explicitly in the name of this compound isA) tert-butyl.
B) methyl.
C) ethyl.
D) propyl.
E) octyl.f.
some other group.
C
4
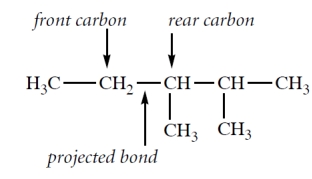
Which one of the Newman projections is correct for the projected bond indicated by the arrow in 2,2,4-trimethylpentane?
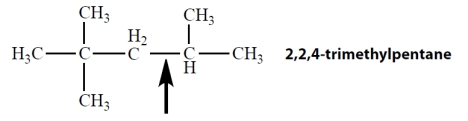
A)
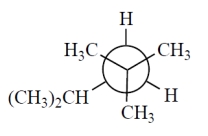
B)
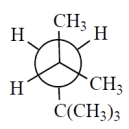
C)
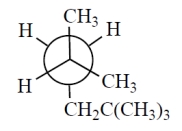

A)

B)

C)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
5
Consider the following alkane, shown in a skeletal structure. What is the IUPAC name of this compound?
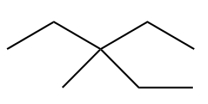


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
6
Consider the alkane, shown in a skeletal structure. Which of the Newman projections depicts one of the staggered conformations of this compound about the projected bond (arrow) when viewed with the carbon marked with a * in the front?
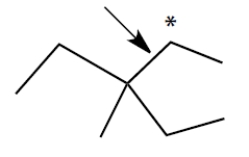
A)
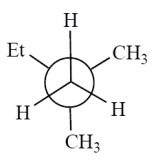
B)
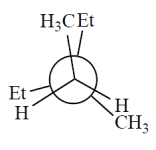
C)
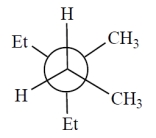
D)
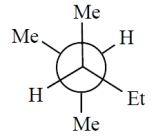

A)

B)

C)

D)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
7
Name the alkane using an IUPAC systematic name.
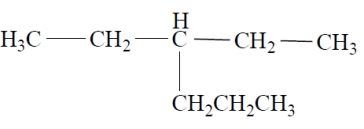


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
8
Provide an IUPAC systematic name for this compound.
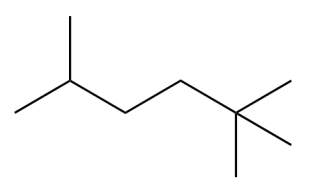


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
9
Consider the compound. This compound is a constitutional isomer of which unbranched alkane?
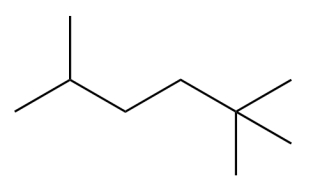


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
10
Complete a Newman projection of the most stable conformation of this compound about the bond marked with the arrow. The carbon with the asterisk should be in front. You can use group abbreviations for large groups.
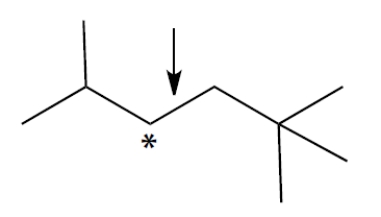


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
11
Which is a correct Newman projection of 3-methylpentane?
A)
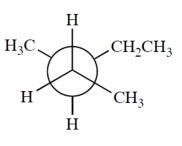
B)
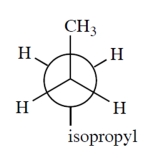
C)
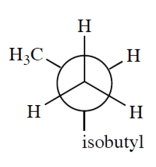
A)

B)

C)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
12
Which compound has the highest boiling point?
A)
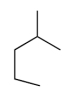
B)
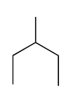
C)
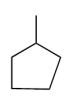
D)
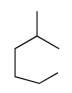
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
13
Consider the IUPAC systematic name of the alkane.
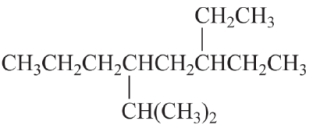
The name of this compound is
A) 5-ethyl-2-methyl-3-propylheptane.
B) 3-ethyl-5-isopropylnonane.
C) 6-ethyl-4-isopropyloctane.
D) 1,1-diethyl-3-isopropylhexane.
E) None of the above is a correct name.
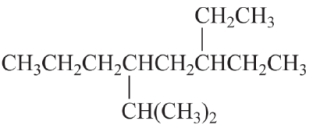
The name of this compound is
A) 5-ethyl-2-methyl-3-propylheptane.
B) 3-ethyl-5-isopropylnonane.
C) 6-ethyl-4-isopropyloctane.
D) 1,1-diethyl-3-isopropylhexane.
E) None of the above is a correct name.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
14
Complete the Newman projection on the right for the compound on the left, with the projection taken about the C3-C4 bond. The Newman projection should show the least stable staggered conformation.
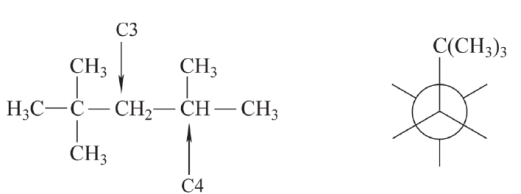


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
15
Behold the structure of vanillin, which is the main ingredient in vanilla extract.
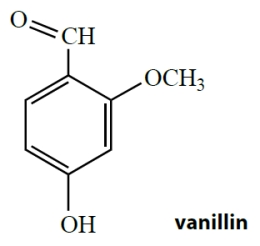
The functional groups in vanillin are best described as
A) a ketone, a phenol, and an ether.
B) an alcohol, an aldehyde, and an ether.
C) a ketone, an alcohol, and an ether.
D) a phenol, an aldehyde, and an ether.
E) an aldehyde, a phenol, and an alcohol.

The functional groups in vanillin are best described as
A) a ketone, a phenol, and an ether.
B) an alcohol, an aldehyde, and an ether.
C) a ketone, an alcohol, and an ether.
D) a phenol, an aldehyde, and an ether.
E) an aldehyde, a phenol, and an alcohol.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
16
Identify the correct name for the compound.
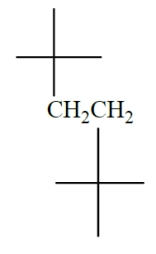
A) 1,1,1,4,4,4-hexamethylbutane
B) 1,2-di-tert-butylethane
C) 2,2,5,5-tetramethylhexane
D) some other name
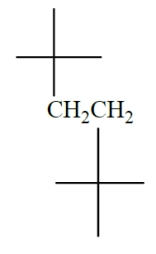
A) 1,1,1,4,4,4-hexamethylbutane
B) 1,2-di-tert-butylethane
C) 2,2,5,5-tetramethylhexane
D) some other name

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
17
Behold the molecule hydroxyproline, drawn in its un-ionized form. Hydroxyproline is an important constituent (in combined form) of collagen. Which correctly describes the functional groups in hydroxyproline?
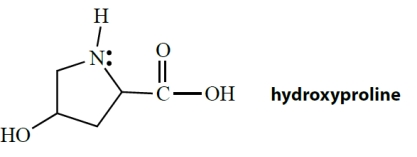
A) alcohol, amine, carboxylic acid
B) alcohol, amide, carboxylic acid
C) amide, amine, carboxylic acid
D) ester, carboxylic acid, amine
E) None of these lists all of the functional groups correctly.

A) alcohol, amine, carboxylic acid
B) alcohol, amide, carboxylic acid
C) amide, amine, carboxylic acid
D) ester, carboxylic acid, amine
E) None of these lists all of the functional groups correctly.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
18
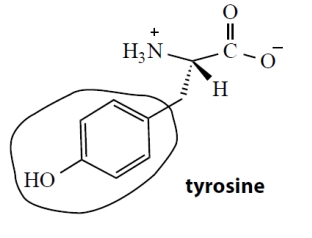
The α-amino acid tyrosine has the following structure. What is the functional group (circled) in the side-chain of tyrosine?


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
19
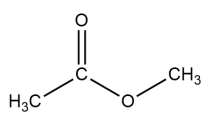
Consider the structure of methyl acetate. Draw an alcohol that is a constitutional isomer. It may contain other functional groups.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
20
The compound with the greatest boiling point is
A)
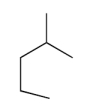
B)
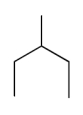
C)
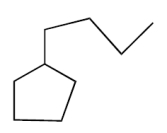
D)
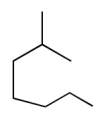
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
21
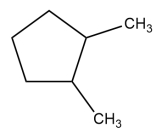
Give the systematic (IUPAC) name of this alkane.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
22
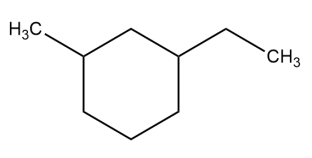
Given the structure, classify each of the carbons as primary, secondary, tertiary, or quaternary.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
23
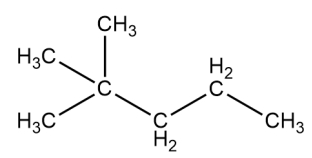
Given the structure, classify each of the hydrogens as primary, secondary, or tertiary.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
24
How many hydrogens are there in a noncyclic alkane containing 10 carbons?

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
25
Draw the structure of 2-butyl-1,1-dimethylcyclohexane.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck


