Deck 10: Free-Radical Reactions, Main-Group Organometallic Compounds, and Carbenes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/25
Play
Full screen (f)
Deck 10: Free-Radical Reactions, Main-Group Organometallic Compounds, and Carbenes
1
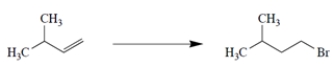
Complete the reaction by giving the missing product.
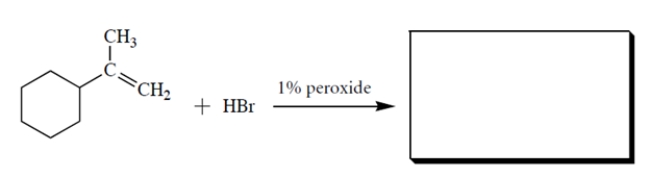

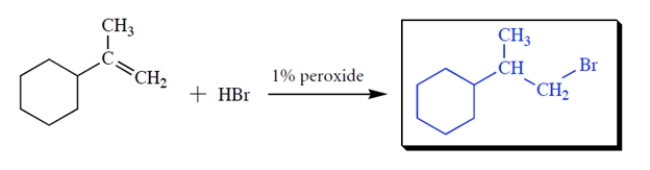
2
Give the structure of the organic free-radical intermediate formed in the propagation steps in the reaction of the alkene with HBr in the presence of peroxides.
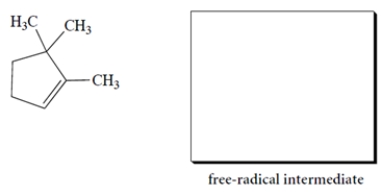

The bromine will add to the less substituted alkene carbon and generate the more stable radical, the tertiary radical. The structure of the free-radical intermediate is
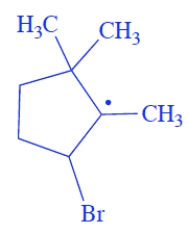

3
Give the product A that results when HBr undergoes peroxide-mediated addition to 1-methylcyclopentene. Also give the structure of the free-radical intermediate B (other than Br.) in the propagation steps of this reaction.

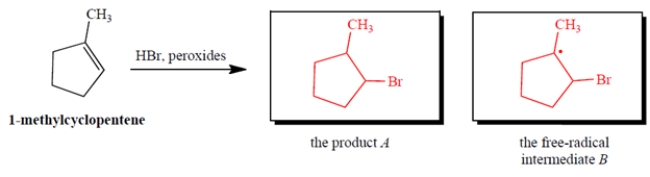
4
Treatment of (+)-(R)-3-methyl-1-pentene with HBr and peroxides yields (-)-1-bromo-3-methyl pentane. Determine the stereochemical configuration of the product.
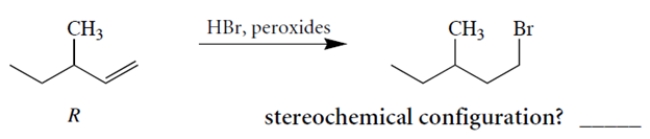


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
5
Thiols (R-S-H) undergo addition to alkenes but only in the presence of peroxides. This equation is an example.
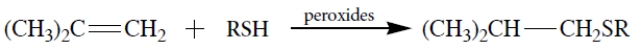 Which one of these is a reactive intermediate in this reaction?
Which one of these is a reactive intermediate in this reaction?
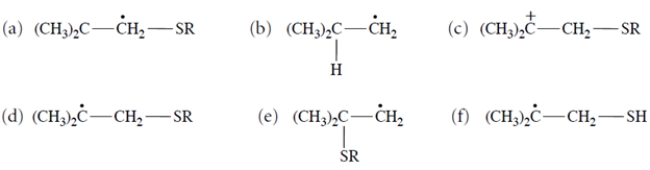
A) compound (a)
B) compound (b)
C) compound (c)
D) compound (d)
E) compound (e)f.
compound (f)
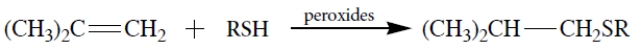 Which one of these is a reactive intermediate in this reaction?
Which one of these is a reactive intermediate in this reaction?
A) compound (a)
B) compound (b)
C) compound (c)
D) compound (d)
E) compound (e)f.
compound (f)

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
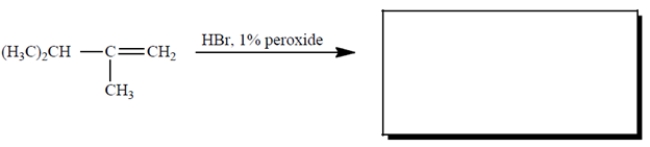
6
Complete the reaction by giving the missing product.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
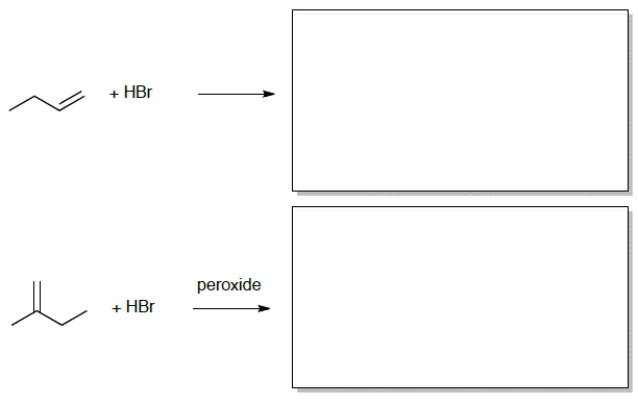
7
Draw the correct product for each of the reactions:


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
8
Complete the reaction by giving the major organic product(s).
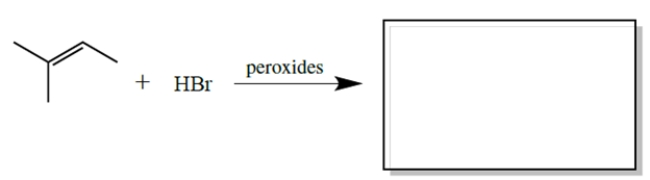


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
9
Consider this addition polymer, called poly(methyl methacrylate). What is the structure of the monomer from which this polymer is formed by free-radical alkene polymerization?
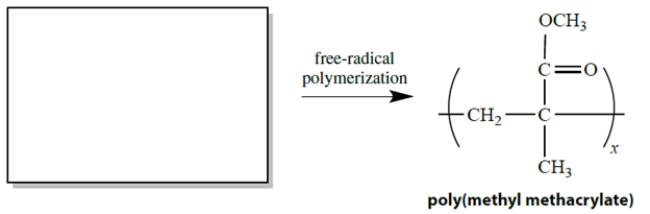


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
10
Complete the reactions by giving the structure of the missing organic products.
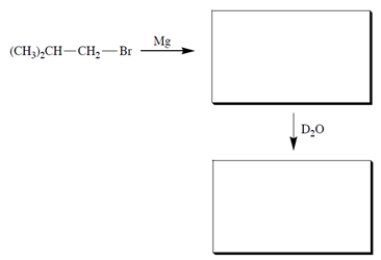


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
11
Complete the reaction by giving the structures of all products.
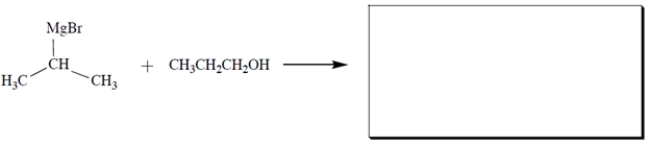


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
12
Provide the structures of the missing compounds.
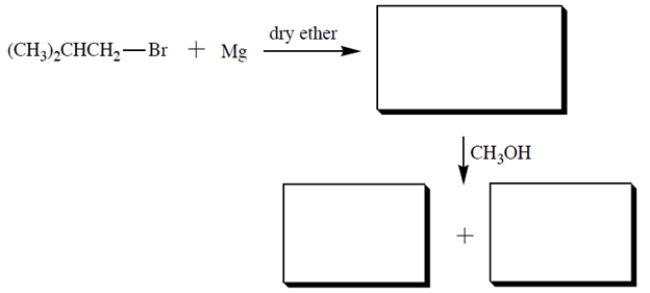


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
13
Which of these compounds is capable of spontaneously providing a free-radical RO• that can initiate free-radical chain reactions?
A) HO-OH
B) (CH3)3O-OC(CH3)3
C) (CH3)3-O-C(CH3)3
D) (CH3)3O-OH
E) All can serve as initiators.
A) HO-OH
B) (CH3)3O-OC(CH3)3
C) (CH3)3-O-C(CH3)3
D) (CH3)3O-OH
E) All can serve as initiators.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
14
Free-radical polymerization of ethylene proceeds with the following propagation steps.
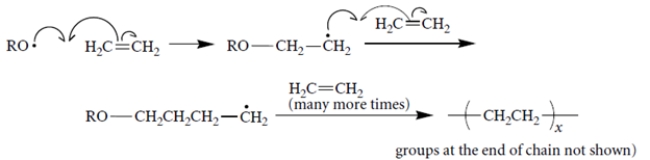 It turns out that polyethylene made by free-radical polymerization contains some branches, for example,
It turns out that polyethylene made by free-radical polymerization contains some branches, for example,
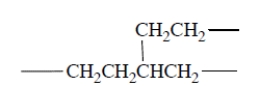 (a) Give a plausible mechanism, complete with "fishhooks," for propagation steps that can account for formation of these branches.
(a) Give a plausible mechanism, complete with "fishhooks," for propagation steps that can account for formation of these branches.
(b) It is possible (by a completely different method) to form polyethylene with unbranched chains. Which do you think would have higher density, branched-chain polyethylene or polyethylene with unbranched chains? Explain briefly.
 It turns out that polyethylene made by free-radical polymerization contains some branches, for example,
It turns out that polyethylene made by free-radical polymerization contains some branches, for example,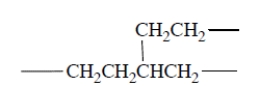 (a) Give a plausible mechanism, complete with "fishhooks," for propagation steps that can account for formation of these branches.
(a) Give a plausible mechanism, complete with "fishhooks," for propagation steps that can account for formation of these branches.(b) It is possible (by a completely different method) to form polyethylene with unbranched chains. Which do you think would have higher density, branched-chain polyethylene or polyethylene with unbranched chains? Explain briefly.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
15
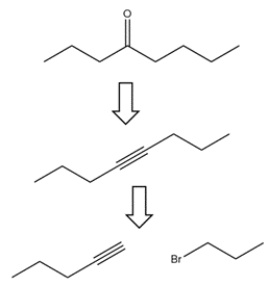
Devise a synthesis to achieve the transformation.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
16
1-Methyl-1-vinyl cyclopentane undergoes addition of HBr under two different conditions as shown in the two pathways. A mixture of products is obtained in Pathway A, whereas a single product is obtained in pathway

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
17
A free-radical addition of a certain compound X-Y to an alkene (R = alkyl) results in the conversion:
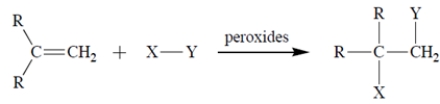 The first propagation step in the mechanism is
The first propagation step in the mechanism is
 Therefore, what is the second propagation step in the mechanism?
Therefore, what is the second propagation step in the mechanism?
A)
B)
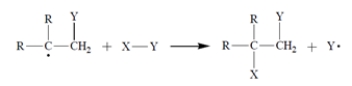
C)

D) Both (b) and (c) are propagation steps.
E) Both (a) and (b) are propagation steps.
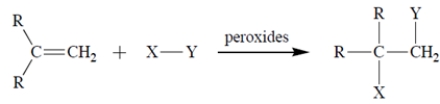 The first propagation step in the mechanism is
The first propagation step in the mechanism is Therefore, what is the second propagation step in the mechanism?
Therefore, what is the second propagation step in the mechanism?A)

B)
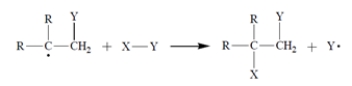
C)

D) Both (b) and (c) are propagation steps.
E) Both (a) and (b) are propagation steps.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
18
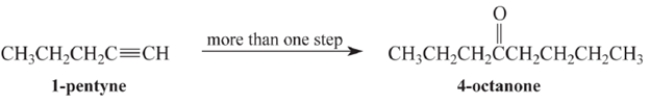
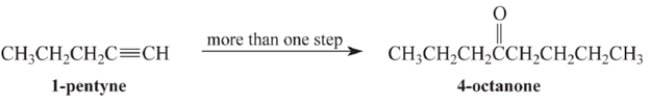
Outline a multi-step synthesis of 4-octanone from 1-pentyne and any other reagents. (Show the reagents required for each step, and the product of each step. Don't show the mechanism.)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
19
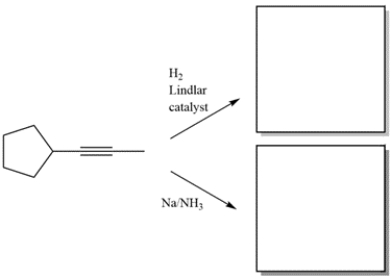
Complete the reactions by giving the structure of the missing organic products.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
20
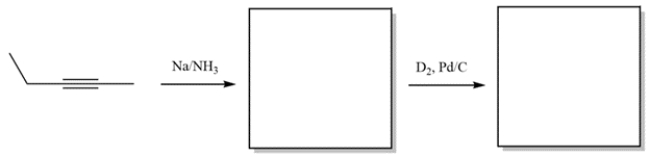
Complete the reactions by giving the structure of the missing organic products.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
21
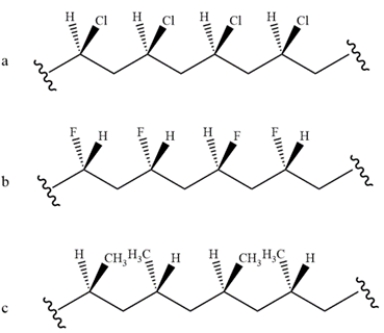
Identify the stereochemistry of the polymers.


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
22
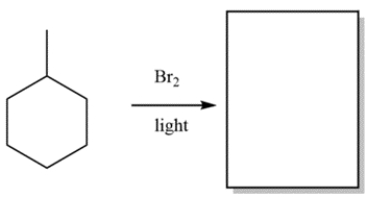
Predict the major organic product of the reaction:


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
23
Outline a synthesis for the alkyne using acetylene and any reagents three carbons or less.
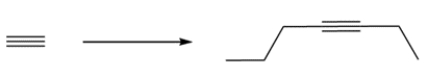


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
24
Outline a synthesis of this product.
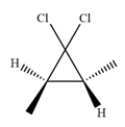


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
25
Provide the major organic product for the reaction.
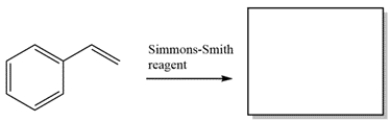


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck


