Deck 3: Proteins
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/52
Play
Full screen (f)
Deck 3: Proteins
1
The number of ways in which protein domains fold in nature is limited. Which of the following is a better estimate of this number?
A) 200
B) 2000
C) 200,000
D) 2,000,000
E) 20 million
A) 200
B) 2000
C) 200,000
D) 2,000,000
E) 20 million
B
Explanation: We know the three-dimensional shapes of over 100,000 proteins. However, most of them fold up into conformations that are not entirely novel, and for which known representative proteins already exist. The number of different protein folds is probably not more than about 2000.
Explanation: We know the three-dimensional shapes of over 100,000 proteins. However, most of them fold up into conformations that are not entirely novel, and for which known representative proteins already exist. The number of different protein folds is probably not more than about 2000.
2
For each of the following cartoon representations from left to right, indicate whether the protein structure is composed of only ? helices (1), only parallel ? sheets (2), only antiparallel ? sheets (3), ? helices plus parallel ? sheets (4), or ? helices plus antiparallel ? sheets (5) as repetitive secondary structure elements. Your answer would be a four-digit number composed of digits 1 to 5 only, e.g. 5513.
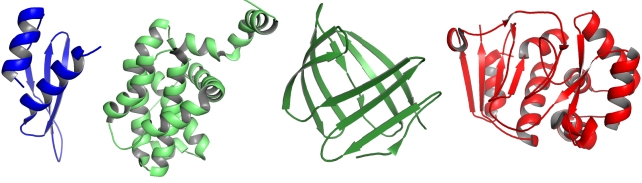
Images created in PyMOL, from PDB entries 1STU, 2LPC, 1SA8, and 3BOB.

Images created in PyMOL, from PDB entries 1STU, 2LPC, 1SA8, and 3BOB.
5134
3
Which of the following is NOT the role of molecular chaperones in the folding of cellular proteins?
A) They assist proteins in folding into their correct conformations.
B) They help prevent formation of protein aggregates.
C) They specify the final three-dimensional shape of proteins.
D) They catalyze the folding of proteins in the crowded environment of the cell.
E) They make the protein-folding process in the cell more reliable.
A) They assist proteins in folding into their correct conformations.
B) They help prevent formation of protein aggregates.
C) They specify the final three-dimensional shape of proteins.
D) They catalyze the folding of proteins in the crowded environment of the cell.
E) They make the protein-folding process in the cell more reliable.
C
Explanation: The molecular chaperones assist a protein in adopting its correct conformation, which is specified by the protein's amino acid sequence.
Explanation: The molecular chaperones assist a protein in adopting its correct conformation, which is specified by the protein's amino acid sequence.
4
You have purified a multisubunit extracellular protein that has several interchain disulfide bonds. Which of the following chemicals would you add to your purified protein mixture if you wanted to eliminate the disulfide bonds?
A) NaCl, a salt
B) SDS, an ionic detergent and denaturing agent
C) H₂O₂, an oxidizing reagent
D) Tris, a buffering agent
E) DTT, a reducing agent
A) NaCl, a salt
B) SDS, an ionic detergent and denaturing agent
C) H₂O₂, an oxidizing reagent
D) Tris, a buffering agent
E) DTT, a reducing agent

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following pairs of amino acid residues would you expect to form ionic bonds?
A) Glutamic acid and glutamine
B) Arginine and lysine
C) Lysine and glutamic acid
D) Tryptophan and tyrosine
E) Tyrosine and glutamine
A) Glutamic acid and glutamine
B) Arginine and lysine
C) Lysine and glutamic acid
D) Tryptophan and tyrosine
E) Tyrosine and glutamine

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
6
Many viruses have large capsids in the form of a hollow sphere, made of hundreds of identical protein subunits. What are the advantages of having coats made of several copies of only a few subunits?
A) Assembly can be readily regulated.
B) Disassembly can be readily regulated.
C) It requires a smaller amount of genetic information.
D) The effect of mistakes in protein synthesis on the overall assembly is minimized.
E) All of the above.
A) Assembly can be readily regulated.
B) Disassembly can be readily regulated.
C) It requires a smaller amount of genetic information.
D) The effect of mistakes in protein synthesis on the overall assembly is minimized.
E) All of the above.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
7
An amino acid residue that is not part of the active site of an enzyme and does not interact with the ligand is nevertheless critical for ligand binding and is highly conserved. How can this be explained?
A) This residue is critical for the correct folding and the placement of ligand-binding site residues.
B) The residue helps with restricting the access of water to the ligand-binding site.
C) This residue can affect the chemical properties of the residues in the ligand-binding site.
D) All of the above.
A) This residue is critical for the correct folding and the placement of ligand-binding site residues.
B) The residue helps with restricting the access of water to the ligand-binding site.
C) This residue can affect the chemical properties of the residues in the ligand-binding site.
D) All of the above.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
8
Imagine a cellular protein composed of 3000 amino acid residues in one continuous polypeptide chain. This protein is almost certainly …
A) extracellular.
B) globular.
C) multidomain.
D) composed of mostly α-helical regions.
E) intrinsically disordered.
A) extracellular.
B) globular.
C) multidomain.
D) composed of mostly α-helical regions.
E) intrinsically disordered.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following stretches of amino acid residues would you expect to find in the interior of protein molecules?
A) Ala-Asp-Asp-Tyr-Arg
B) Gly-Lys-Ser-Pro-Thr
C) Phe-Glu-Gln-Glu-Asn
D) Ala-Val-Leu-Ile-Trp
E) Gly-Tyr-His-Arg-His
A) Ala-Asp-Asp-Tyr-Arg
B) Gly-Lys-Ser-Pro-Thr
C) Phe-Glu-Gln-Glu-Asn
D) Ala-Val-Leu-Ile-Trp
E) Gly-Tyr-His-Arg-His

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
10
At physiological pH, what is the approximate net charge of a hexapeptide with the following amino acid sequence?
Asp-Val-Ile-Glu-Arg-Ser
A) -2
B) -1
C) 0
D) +1
E) +2
Asp-Val-Ile-Glu-Arg-Ser
A) -2
B) -1
C) 0
D) +1
E) +2

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is NOT true regarding the members of a protein family in general?
A) They have similar three-dimensional conformations.
B) They share an ancestry; i.e. they are homologs.
C) They can functionally replace each other.
D) Their gene sequence is less well conserved than their structure.
E) Over evolutionary time scales, the family has expanded mainly through gene duplication events.
A) They have similar three-dimensional conformations.
B) They share an ancestry; i.e. they are homologs.
C) They can functionally replace each other.
D) Their gene sequence is less well conserved than their structure.
E) Over evolutionary time scales, the family has expanded mainly through gene duplication events.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
12
Evolutionary tracing has identified clusters of the most invariant amino acids in the SH2 domain family. Which of the following is true regarding these sites?
A) Mutation in these sites generally keeps the protein functional.
B) The invariant amino acids generally have negatively charged side chains.
C) These sites correspond to the binding site for peptides containing phosphorylated tyrosine.
D) The amino acids at these sites are generally hydrophobic.
E) These sites have evolved fast because they have critical functions.
A) Mutation in these sites generally keeps the protein functional.
B) The invariant amino acids generally have negatively charged side chains.
C) These sites correspond to the binding site for peptides containing phosphorylated tyrosine.
D) The amino acids at these sites are generally hydrophobic.
E) These sites have evolved fast because they have critical functions.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
13
We can try to map the surface of a globular protein in two dimensions just like we depict the surface of the Earth in a world map. On such a map, the binding site of a molecule for other molecules of the same protein (for assembly into a multisubunit complex) can be marked. In the following "maps," two molecules of the protein can bind if the points a, b, and c on the surface of one molecule can align to points a′, b′, and c′ in the other molecule, respectively. Which map corresponds to a protein whose assembly gives rise to a helix? Hint: the other maps correspond to a dimer, a ring, and a linear filament. 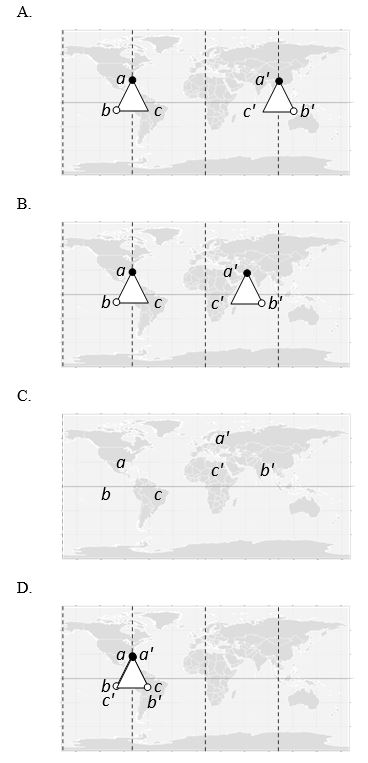


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
14
Stable β-sheet aggregates can form from many proteins, forming intertwined cross-beta strands that have the potential to kill cells or damage tissues. Which of the following is NOT true regarding these aggregates?
A) They form almost exclusively in the cells of the nervous system.
B) Different types of such aggregates can form from the same protein.
C) Their formation is associated with conditions such as Parkinson's disease and Kuru.
D) They can form spontaneously, but also can be triggered to form by an infection with the same aggregate.
E) Some healthy cells form these aggregates to store their secretory proteins.
A) They form almost exclusively in the cells of the nervous system.
B) Different types of such aggregates can form from the same protein.
C) Their formation is associated with conditions such as Parkinson's disease and Kuru.
D) They can form spontaneously, but also can be triggered to form by an infection with the same aggregate.
E) Some healthy cells form these aggregates to store their secretory proteins.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
15
The human estrogen receptor is a symmetrical dimeric nuclear protein that can regulate gene expression by binding to a DNA sequence called an estrogen response element (ERE) near the promoter of its target genes. Each subunit of the receptor binds to about six base pairs of DNA. Which of the following sequences is a likely candidate for the ERE? The sequences are written in the 5′-to-3′ direction. The letter N represents any of the four DNA bases.
A) AGGTCANNNTGACCT
B) AGGTCANNNAGGTCA
C) AGGTCANNNACTGGA
D) AGGTCANNNATATAT
E) AGGCCTNNNTCATGA
A) AGGTCANNNTGACCT
B) AGGTCANNNAGGTCA
C) AGGTCANNNACTGGA
D) AGGTCANNNATATAT
E) AGGCCTNNNTCATGA

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
16
Protein A can bind to each of the proteins B or C. The association rate constants are the same for forming the AB and the AC complexes. However, the dissociation rate constant for AB is 100 times higher than that for AC. Given that every tenfold increase in the equilibrium constant (of the association reaction) corresponds to about -5.9 kJ/mole difference in the standard free-energy change for the reaction (ΔG°), what is the value of (ΔG°AB - ΔG°AC) in kJ/mole?
A) -11.8
B) -5.9
C) 0
D) +5.9
D) +5.9
A) -11.8
B) -5.9
C) 0
D) +5.9
D) +5.9

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
17
The observation that proteins often renature into their original conformations after they have been unfolded by denaturing solvents implies that …
A) the information needed to specify the three-dimensional shape of a protein is encoded in its amino acid sequence.
B) the cell does not need molecular chaperones for survival.
C) the final folded structure of a protein is usually NOT the one with the lowest free energy.
D) each protein folds into several different conformations inside the cell.
E) All of the above.
A) the information needed to specify the three-dimensional shape of a protein is encoded in its amino acid sequence.
B) the cell does not need molecular chaperones for survival.
C) the final folded structure of a protein is usually NOT the one with the lowest free energy.
D) each protein folds into several different conformations inside the cell.
E) All of the above.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
18
Protein secondary structure elements such as α helices and β sheets constitute the major regular folding patterns in proteins. With regard to these elements, …
A) hydrogen-bonding between the amino acid side chains defines the type of secondary structure.
B) a certain short amino acid sequence always adopts the same secondary structure.
C) only a few specific amino acid sequences can adopt these repetitive structures.
D) the folding patterns result from hydrogen-bonding between the N-H and C=O groups in the polypeptide backbone.
E) All of the above.
A) hydrogen-bonding between the amino acid side chains defines the type of secondary structure.
B) a certain short amino acid sequence always adopts the same secondary structure.
C) only a few specific amino acid sequences can adopt these repetitive structures.
D) the folding patterns result from hydrogen-bonding between the N-H and C=O groups in the polypeptide backbone.
E) All of the above.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
19
In interactions between proteins, each hydrogen bond contributes to the free energy of binding by about -4 kJ/mole. If two proteins bind to each other through nine hydrogen bonds, six of which are eliminated when one of these proteins is mutated, how much would you expect the equilibrium constant for their binding to change as a result of the mutation? (ΔG° = -5.9 × log Kₑq)
A) Increase sixfold
B) Decrease fourfold
C) Decrease by six orders of magnitude
D) Increase 1 million-fold
E) Decrease by four orders of magnitude
A) Increase sixfold
B) Decrease fourfold
C) Decrease by six orders of magnitude
D) Increase 1 million-fold
E) Decrease by four orders of magnitude

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following can be a function for intrinsically disordered protein sequences?
A) High-specificity binding to other proteins
B) Cell signaling through covalent modification of the protein sequence
C) Tethering to hold interacting proteins in close proximity
D) Formation of a diffusion barrier from a dense network of such sequences
E) All of the above
A) High-specificity binding to other proteins
B) Cell signaling through covalent modification of the protein sequence
C) Tethering to hold interacting proteins in close proximity
D) Formation of a diffusion barrier from a dense network of such sequences
E) All of the above

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
21
In terms of molecular function, what do Ras and myosin have in common?
A) They normally bind to GTP.
B) They couple the hydrolysis of a bound nucleoside triphosphate to protein movements.
C) They switch between two distinct conformations controlled by protein phosphorylation and dephosphorylation.
D) They are multisubunit proteins and contain a scaffold subunit.
E) All of the above.
A) They normally bind to GTP.
B) They couple the hydrolysis of a bound nucleoside triphosphate to protein movements.
C) They switch between two distinct conformations controlled by protein phosphorylation and dephosphorylation.
D) They are multisubunit proteins and contain a scaffold subunit.
E) All of the above.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
22
How do multienzyme complexes in the cell, such as the fatty acid synthase, enhance reaction rates?
A) By increasing the diffusion rate of their substrate in the cell
B) By allowing the channeling of pathway intermediates from one enzyme to the next
C) By limiting the diffusion of the substrates in membrane-bound compartments
D) By increasing the intrinsic rate of catalysis for individual enzymes
E) All of the above
A) By increasing the diffusion rate of their substrate in the cell
B) By allowing the channeling of pathway intermediates from one enzyme to the next
C) By limiting the diffusion of the substrates in membrane-bound compartments
D) By increasing the intrinsic rate of catalysis for individual enzymes
E) All of the above

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
23
Switch proteins that bind and hydrolyze GTP are ubiquitous cell regulators in a wide variety of molecular processes. Which of the following statements is NOT true regarding these proteins?
A) EF-Tu is an example of such proteins.
B) GTP hydrolysis by the GTPase generally renders it inactive.
C) Activation of the GTPase involves addition of a phosphate group to its bound guanine nucleotide.
D) Guanine nucleotide exchange factors generally activate the GTPases.
E) Guanine nucleotide exchange factors accelerate the release of bound GDP from the GTPases.
A) EF-Tu is an example of such proteins.
B) GTP hydrolysis by the GTPase generally renders it inactive.
C) Activation of the GTPase involves addition of a phosphate group to its bound guanine nucleotide.
D) Guanine nucleotide exchange factors generally activate the GTPases.
E) Guanine nucleotide exchange factors accelerate the release of bound GDP from the GTPases.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
24
The compound GDPNP is a GTP analog that can bind to GTPases in the same way as GTP. However, unlike GTP, it cannot undergo the hydrolysis reaction that normally releases Pᵢ. Therefore, …
A) GDPNP and GDP bind to the same conformation of a GTPase.
B) a GDPNP-bound Ras is constitutively active.
C) a GDPNP-bound EF-Tu cannot bind to tRNA molecules.
D) binding of GDPNP to EF-Tu allows multiple cycles of tRNA binding and release without the use of free energy.
E) None of the above.
A) GDPNP and GDP bind to the same conformation of a GTPase.
B) a GDPNP-bound Ras is constitutively active.
C) a GDPNP-bound EF-Tu cannot bind to tRNA molecules.
D) binding of GDPNP to EF-Tu allows multiple cycles of tRNA binding and release without the use of free energy.
E) None of the above.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
25
Many macromolecular complexes in the cell contain scaffold proteins. What do these proteins do that benefits the cell?
A) They can enhance the rate of critical cellular reactions.
B) They can hold the many subunits of a large complex together.
C) They can confine and concentrate a specific set of interacting proteins to a particular cellular location.
D) They can provide a large macromolecular complex with either flexibility or rigidity.
E) All of the above.
A) They can enhance the rate of critical cellular reactions.
B) They can hold the many subunits of a large complex together.
C) They can confine and concentrate a specific set of interacting proteins to a particular cellular location.
D) They can provide a large macromolecular complex with either flexibility or rigidity.
E) All of the above.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
26
The enzyme lysozyme catalyzes the cutting of a polysaccharide chain through hydrolysis. Which of the following is NOT true regarding the catalytic cycle for this enzyme?
A) It involves acid catalysis.
B) It involves base catalysis.
C) It involves strain catalysis.
D) It involves covalent catalysis.
E) It involves metal ion catalysis.
A) It involves acid catalysis.
B) It involves base catalysis.
C) It involves strain catalysis.
D) It involves covalent catalysis.
E) It involves metal ion catalysis.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
27
The phosphofructokinase (PFK) enzyme is one of the key players in the glycolytic pathway in which glucose eventually breaks down into pyruvate, some ATP is generated, and some NAD⁺ is reduced. PFK catalyzes the committed step in the pathway and is under extensive regulation. Which of the following compounds would you expect to activate PFK?
A) ADP
B) NADH
C) Pyruvate
D) ATP
E) All of the above
A) ADP
B) NADH
C) Pyruvate
D) ATP
E) All of the above

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
28
The turnover number for an enzyme is equivalent to the number of substrate molecules processed per second per enzyme molecule. To a test tube containing a 100 mM concentration of its substrate, you have added an enzyme at a final concentration of 10 µM, and have measured the rate of the reaction to be approximately 500 µM/sec. If the Km for the binding of the enzyme to this substrate is about 100 mM, what is the turnover number?
A) 100
B) 500
C) 1000
D) 5000
E) 10,000
A) 100
B) 500
C) 1000
D) 5000
E) 10,000

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
29
Enzymes can catalyze cellular reactions through various mechanisms. Which of the following statements is NOT true regarding enzymes?
A) They can provide the chemical groups necessary for simultaneous acid and base catalysis.
B) They have a higher affinity for the transition state of the substrate than for its stable form.
C) They can form covalent bonds with the substrate during catalysis.
D) They can strain a substrate to force it toward a specific transition state.
E) They accelerate a cellular reaction by destabilizing the transition state.
A) They can provide the chemical groups necessary for simultaneous acid and base catalysis.
B) They have a higher affinity for the transition state of the substrate than for its stable form.
C) They can form covalent bonds with the substrate during catalysis.
D) They can strain a substrate to force it toward a specific transition state.
E) They accelerate a cellular reaction by destabilizing the transition state.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
30
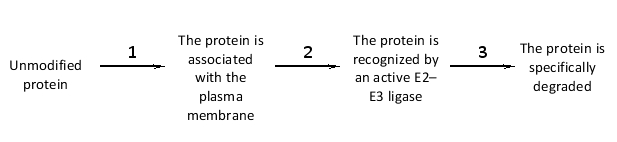
The marking of a protein by polyubiquitylation to signify degradation …
A) requires the hydrolysis of one ATP molecule to ADP per polyubiquitin chain.
B) involves covalent attachment of the target protein to the E1 enzyme.
C) is carried out by the proteasome complex.
D) is typically done on an arginine residue in the target protein.
E) involves the recognition of the target protein by an E2-E3 ligase.
A) requires the hydrolysis of one ATP molecule to ADP per polyubiquitin chain.
B) involves covalent attachment of the target protein to the E1 enzyme.
C) is carried out by the proteasome complex.
D) is typically done on an arginine residue in the target protein.
E) involves the recognition of the target protein by an E2-E3 ligase.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
31
The SCF ubiquitin ligase can recognize and mark various target proteins at different stages of the cell cycle. In this complex, …
A) different F-box subunits recognize different target proteins.
B) the F-box subunit and the E2 ubiquitin-conjugating enzyme are at the opposite ends of the C-shaped molecule.
C) a scaffold protein arranges the other subunits such that the two ends of the complex are separated by a gap.
D) the use of interchangeable parts such as the F-box subunits makes economical use of the genetic information and allows for rapid evolution of new functions.
E) All of the above.
A) different F-box subunits recognize different target proteins.
B) the F-box subunit and the E2 ubiquitin-conjugating enzyme are at the opposite ends of the C-shaped molecule.
C) a scaffold protein arranges the other subunits such that the two ends of the complex are separated by a gap.
D) the use of interchangeable parts such as the F-box subunits makes economical use of the genetic information and allows for rapid evolution of new functions.
E) All of the above.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
32
A polyubiquitin chain has been attached to a protein. The ubiquitin molecules are linked together via isopeptide bonds between Lys48 of one molecule and the carboxyl end of the next one. This protein is expected to …
A) be a part of chromatin.
B) undergo proteasomal degradation.
C) be involved in DNA repair.
D) be targeted to endocytic vesicles.
E) None of the above.
A) be a part of chromatin.
B) undergo proteasomal degradation.
C) be involved in DNA repair.
D) be targeted to endocytic vesicles.
E) None of the above.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
33
Consider the proteins Ras, Src, kinesin, and the ATP synthase pump. All of them …
A) are allosteric proteins.
B) can have two or more distinct conformations.
C) can bind to ATP (or GTP).
D) can hydrolyze ATP to ADP (or GTP to GDP).
E) All of the above.
A) are allosteric proteins.
B) can have two or more distinct conformations.
C) can bind to ATP (or GTP).
D) can hydrolyze ATP to ADP (or GTP to GDP).
E) All of the above.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
34
Most GTPases are present inside the cell at a much higher concentration than their upstream GAP and GEF proteins. Imagine a mutation in a certain GTPase, such as a Rab protein, resulting in an extremely tight binding between the GTPase and its GEF, and a very slow dissociation. What would you expect to happen in the cell as a result?
A) Rab proteins will be activated, because the tightly bound GEFs will be unavailable.
B) Rab proteins will be inactivated, because the tightly bound GEFs will be unavailable.
C) Rab proteins will be activated, because the Rab-GAPs will become activated.
D) Rab proteins will be inactivated, because their GAPs will become activated.
E) Rab proteins will be activated, because there are fewer GAPs available.
A) Rab proteins will be activated, because the tightly bound GEFs will be unavailable.
B) Rab proteins will be inactivated, because the tightly bound GEFs will be unavailable.
C) Rab proteins will be activated, because the Rab-GAPs will become activated.
D) Rab proteins will be inactivated, because their GAPs will become activated.
E) Rab proteins will be activated, because there are fewer GAPs available.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the regulatory interactions 1 to 5 depicted in the following diagram is NOT an example of a negative feedback regulation? 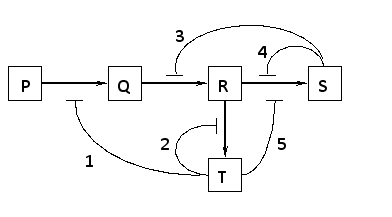
A) 1
B) 2
C) 3
D) 4
E) 5

A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
36
Phosphorylation of a protein by a protein kinase …
A) adds two positive charges to the protein.
B) activates the protein.
C) deactivates the protein.
D) can create a binding site for other proteins.
E) requires the hydrolysis of two molecules of ATP per phosphorylated residue.
A) adds two positive charges to the protein.
B) activates the protein.
C) deactivates the protein.
D) can create a binding site for other proteins.
E) requires the hydrolysis of two molecules of ATP per phosphorylated residue.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
37
In the following schematic diagram of a simple signaling pathway, protein Z regulates the activity of protein X, which is an upstream protein kinase, through a negative feedback loop. Which of the following better describes protein Z? 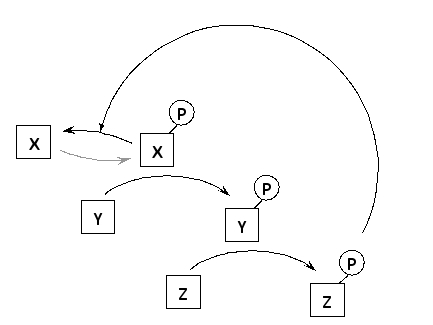
A) It is a protein kinase that is activated by phosphorylation.
B) It is a protein kinase that is inactivated by phosphorylation.
C) It is a protein phosphatase that is activated by phosphorylation.
D) It is a protein phosphatase that is inactivated by phosphorylation.

A) It is a protein kinase that is activated by phosphorylation.
B) It is a protein kinase that is inactivated by phosphorylation.
C) It is a protein phosphatase that is activated by phosphorylation.
D) It is a protein phosphatase that is inactivated by phosphorylation.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
38
An enzyme acts on a tyrosine residue in a target protein to create a binding site for the SH2 domain. This enzyme is most specifically …
A) an isomerase.
B) a nuclease.
C) a phosphatase.
D) a kinase.
E) a synthase.
A) an isomerase.
B) a nuclease.
C) a phosphatase.
D) a kinase.
E) a synthase.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
39
A viral version of the Src kinase called v-Src is found in some retroviruses. Unlike the cellular Src, the v-Src kinase is constitutively active, and can drive the cell into uncontrolled growth and tumor formation. Which of the following molecular differences between the two versions of Src is more likely to be responsible for this?
A) v-Src lacks the active-site tyrosine residue.
B) v-Src lacks a lobe of the kinase domain.
C) v-Src has multiple inhibitory tyrosine phosphorylation sites in the kinase domain.
D) v-Src lacks the C-terminal tail that can bind to Src's SH2 domain.
E) v-Src has a longer C-terminal tail.
A) v-Src lacks the active-site tyrosine residue.
B) v-Src lacks a lobe of the kinase domain.
C) v-Src has multiple inhibitory tyrosine phosphorylation sites in the kinase domain.
D) v-Src lacks the C-terminal tail that can bind to Src's SH2 domain.
E) v-Src has a longer C-terminal tail.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
40
Two ligands, A and B, bind to two different conformations of the enzyme X. The ligand A is the enzyme's substrate, whereas ligand B binds to a remote allosteric site. Which of the following is a consequence of this arrangement?
A) Binding of A to X does not affect the affinity of X for binding to B.
B) Binding of B to X does not affect the rate of reaction catalyzed by X.
C) Binding of A to X increases the affinity of X for B.
D) Binding of B to X decreases the affinity of X for A.
E) Binding of B to X has a large effect on the binding of A to X, but binding of A to X has a small effect on X-B binding.
A) Binding of A to X does not affect the affinity of X for binding to B.
B) Binding of B to X does not affect the rate of reaction catalyzed by X.
C) Binding of A to X increases the affinity of X for B.
D) Binding of B to X decreases the affinity of X for A.
E) Binding of B to X has a large effect on the binding of A to X, but binding of A to X has a small effect on X-B binding.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
41
Imagine a protein that can be independently phosphorylated on any of its 10 tyrosine residues and acetylated on either of its 2 lysine residues. In principle, how many different combinations of these modifications are possible for this protein?
A) 2¹⁰
B) 2²⁰
C) 10¹⁰
D) 12²⁰
E) 2¹²
A) 2¹⁰
B) 2²⁰
C) 10¹⁰
D) 12²⁰
E) 2¹²

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
42
The structural formulas for the 20 naturally occurring amino acid residues are shown in the panel below. Answer the following question(s) with the help of this panel.
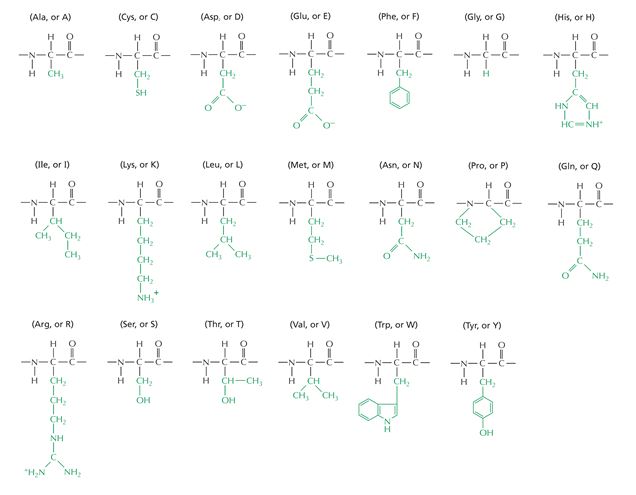
-Which of the amino acids shown above has the most limited combinations of phi (?) and psi (?) angles in its Ramachandran plot? Write down the one-letter abbreviation for it, e.g. A.

-Which of the amino acids shown above has the most limited combinations of phi (?) and psi (?) angles in its Ramachandran plot? Write down the one-letter abbreviation for it, e.g. A.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
43
A protein is drawn in the following simplified diagram undergoing a set of covalent modifications including the addition of a chain of ubiquitin protein monomers (U) to one of its lysine side chains, a phosphate moiety (P) to its tyrosine side chain, and a lipid farnesyl (F) to its cysteine side chain. Indicate the order of these modifications (1 to 3) in the diagram. Your answer would be a three-letter string composed of letters F, U, and P only, e.g. PFU.
Answers

Answers

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
44
The structural formulas for the 20 naturally occurring amino acid residues are shown in the panel below. Answer the following question(s) with the help of this panel.

-What is the amino acid sequence of the peptide depicted below? Write down the sequence from the N- to the C-terminus, and use only the one-letter abbreviations, e.g. AGCNT.
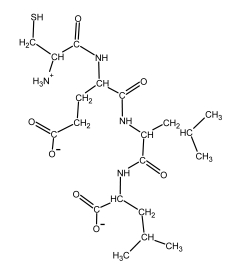

-What is the amino acid sequence of the peptide depicted below? Write down the sequence from the N- to the C-terminus, and use only the one-letter abbreviations, e.g. AGCNT.


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
45
In an ? helix, each amino acid residue is rotated by about +100° and raised by about 0.15 nm relative to the previous residue. This means that each full turn of the helix rises by … nm parallel to the helix's axis.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
46
Imagine that 1 L of a solution containing each of the 20 naturally occurring amino acids at 50 mM concentration each (total concentration of 1 M) is allowed to polymerize in a perfectly stepwise fashion such that at each step, a random amino acid can be incorporated into a growing polypeptide. The steps are repeated, until eventually the solution is only composed of 40-mers (and virtually all of the monomers have been used). What fraction of all 40-mers can possibly be present in this solution? Round your number to four decimal places. Avogadro's number is 6 × 10²³.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
47
For a simple enzymatic reaction that involves only one substrate and follows Michaelis-Menten kinetics, the changes in the concentrations of substrate, product, free enzyme, and the enzyme-substrate complex over the course of the reaction are depicted by solid curves in the graph below. Which curve (1 to 4) corresponds to [ES]? Write down the number as your answer.
![For a simple enzymatic reaction that involves only one substrate and follows Michaelis-Menten kinetics, the changes in the concentrations of substrate, product, free enzyme, and the enzyme-substrate complex over the course of the reaction are depicted by solid curves in the graph below. Which curve (1 to 4) corresponds to [ES]? Write down the number as your answer.](https://storage.examlex.com/TB1030/11ea33de_1b49_f77b_bddc_e7510d8256b9_TB1030_00.jpg)
![For a simple enzymatic reaction that involves only one substrate and follows Michaelis-Menten kinetics, the changes in the concentrations of substrate, product, free enzyme, and the enzyme-substrate complex over the course of the reaction are depicted by solid curves in the graph below. Which curve (1 to 4) corresponds to [ES]? Write down the number as your answer.](https://storage.examlex.com/TB1030/11ea33de_1b49_f77b_bddc_e7510d8256b9_TB1030_00.jpg)

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
48
The structural formulas for the 20 naturally occurring amino acid residues are shown in the panel below. Answer the following question(s) with the help of this panel.

-What is the amino acid sequence of the peptide depicted below? Write down the sequence from the N- to the C-terminus, and use only the one-letter abbreviations, e.g. AGCNT.
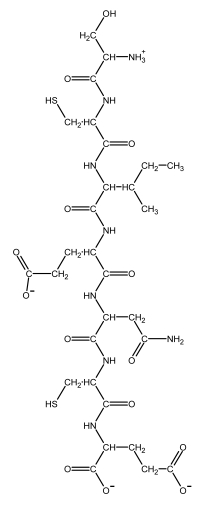

-What is the amino acid sequence of the peptide depicted below? Write down the sequence from the N- to the C-terminus, and use only the one-letter abbreviations, e.g. AGCNT.


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
49
A simple protein interaction map is shown below for human cytochrome c (Cyt), a heme-containing protein that is normally found inside the mitochondria and is associated with the electron-transport chain (ETC) of the inner mitochondrial membrane. Under special emergency conditions, this protein can also moonlight as a signal transducer for the onset of a pathway leading to programmed cell death (PCD). As shown in the map, it also interacts with a group of phosphoprotein phosphatases (PPP) involved in cell signaling pathways. Assuming that the functions of the proteins labeled as X, Y, and Z are unknown, which of the following points can be reasonably argued from this interaction map? 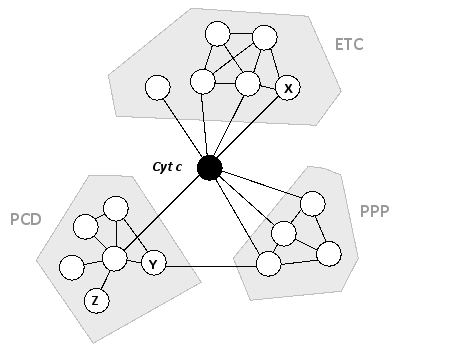
A) The protein labeled X probably functions in PCD as well as in the ETC, because it interacts with cytochrome c, which is known to be closely involved in PCD, and it also interacts with several proteins from the ETC group.
B) The protein labeled Y probably has a role in PCD, especially if its orthologs in other organisms have such a role and show a similar pattern of interactions.
C) If the proteins in the PCD group are known to form a large complex, then the protein labeled Z is likely to be the scaffold protein for that complex.
D) The protein labeled Y probably has a phosphatase function, because it interacts with the proteins of the PPP group.
E) The protein labeled Z is probably not an essential protein because it only interacts with one other protein in this map.

A) The protein labeled X probably functions in PCD as well as in the ETC, because it interacts with cytochrome c, which is known to be closely involved in PCD, and it also interacts with several proteins from the ETC group.
B) The protein labeled Y probably has a role in PCD, especially if its orthologs in other organisms have such a role and show a similar pattern of interactions.
C) If the proteins in the PCD group are known to form a large complex, then the protein labeled Z is likely to be the scaffold protein for that complex.
D) The protein labeled Y probably has a phosphatase function, because it interacts with the proteins of the PPP group.
E) The protein labeled Z is probably not an essential protein because it only interacts with one other protein in this map.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
50
Some protein domains are found in many different proteins and have been especially mobile during evolution. A domain of this kind is called a "protein …"

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
51
The structural formulas for the 20 naturally occurring amino acid residues are shown in the panel below. Answer the following question(s) with the help of this panel.

-What is the amino acid sequence of the peptide depicted below? Write down the sequence from the N- to the C-terminus, and use only the one-letter abbreviations, e.g. AGCNT.
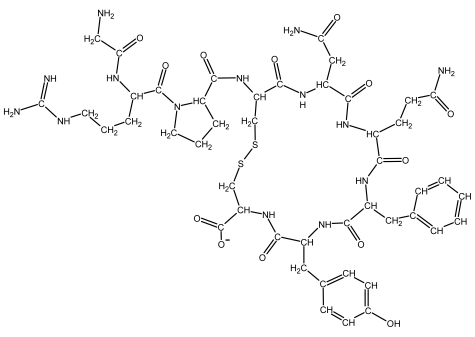

-What is the amino acid sequence of the peptide depicted below? Write down the sequence from the N- to the C-terminus, and use only the one-letter abbreviations, e.g. AGCNT.


Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck
52
The structural formulas for the 20 naturally occurring amino acid residues are shown in the panel below. Answer the following question(s) with the help of this panel.

-Which amino acid residues shown above have acidic side chains? Write down the one-letter abbreviations for them, in alphabetical order, e.g. ACGNV.

-Which amino acid residues shown above have acidic side chains? Write down the one-letter abbreviations for them, in alphabetical order, e.g. ACGNV.

Unlock Deck
Unlock for access to all 52 flashcards in this deck.
Unlock Deck
k this deck


