Deck 4: Systems of Measurement
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/20
Play
Full screen (f)
Deck 4: Systems of Measurement
1
Which of the following statements is true?
A) Each increment on the Kelvin scale is considered 1°K.
B) Each increment on the Kelvin scale is equal to °C + 100.
C) Each increment on the Kelvin scale corresponds to the absence of thermal energy.
D) Each increment on the Kelvin scale is the same as an increment on the Celsius scale.
A) Each increment on the Kelvin scale is considered 1°K.
B) Each increment on the Kelvin scale is equal to °C + 100.
C) Each increment on the Kelvin scale corresponds to the absence of thermal energy.
D) Each increment on the Kelvin scale is the same as an increment on the Celsius scale.
Each increment on the Kelvin scale is the same as an increment on the Celsius scale.
2
When converting between units,which of the following should be done when moving to a larger unit?
A) Add
B) Divide
C) Multiply
D) Subtract
A) Add
B) Divide
C) Multiply
D) Subtract
Divide
3
Which of the following is NOT an accepted temperature scale?
A) Celsius
B) Degrees
C) Fahrenheit
D) Kelvin
A) Celsius
B) Degrees
C) Fahrenheit
D) Kelvin
Degrees
4
Which of the following is true regarding the unit "mole"?
A) If the masses of two atoms differ, then a mole of one substance will weigh more than a mole of the other.
B) Because the number of atoms in a mole is constant, one mole of any substance has the same weight as does one mole of any other substance.
C) The unit "mole" uses the standard of one mole of carbon-12, because one mole of carbon-12 is the heaviest substance known.
D) Both A and C are correct.
A) If the masses of two atoms differ, then a mole of one substance will weigh more than a mole of the other.
B) Because the number of atoms in a mole is constant, one mole of any substance has the same weight as does one mole of any other substance.
C) The unit "mole" uses the standard of one mole of carbon-12, because one mole of carbon-12 is the heaviest substance known.
D) Both A and C are correct.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
5
When using Approach 2 to convert metric system units,if the conversion is going toward the smaller unit which of the following is true?
A) The value should be added to the ratio of the larger factor to the smaller factor.
B) The value should be divided by the ratio of the larger factor to the smaller factor.
C) The value should be multiplied by the ratio of the larger factor to the smaller factor.
D) The value should be subtracted from the ratio of the larger factor to the smaller factor.
A) The value should be added to the ratio of the larger factor to the smaller factor.
B) The value should be divided by the ratio of the larger factor to the smaller factor.
C) The value should be multiplied by the ratio of the larger factor to the smaller factor.
D) The value should be subtracted from the ratio of the larger factor to the smaller factor.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
6
At what temperature does water freeze on the Celsius temperature scale?
A) 0°
B) 32°
C) 72°
D) 100°
A) 0°
B) 32°
C) 72°
D) 100°

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
7
How does one use the ratio method to convert units?
A) Calculate the ratio of the known value to the value of the desired value.
B) Create a ratio of the known units divided by the desired units and determine the dividend.
C) Determine the ratio of the value in known units to the equivalent of the value in desired units.
D) Set up an equation of ratios and then cross multiply the values and units.
A) Calculate the ratio of the known value to the value of the desired value.
B) Create a ratio of the known units divided by the desired units and determine the dividend.
C) Determine the ratio of the value in known units to the equivalent of the value in desired units.
D) Set up an equation of ratios and then cross multiply the values and units.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
8
What system of units includes liter and meter?
A) International System of Units
B) Metric System
C) United States Customary System of Units
D) United States Standards for Units and Measurements
A) International System of Units
B) Metric System
C) United States Customary System of Units
D) United States Standards for Units and Measurements

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
9
What is a mole?
A) The amount of a substance that consists of as many entities as there are atoms in exactly 12 grams of the element carbon-12
B) The number of atoms of a substance found in 10 mL of the substance
C) The total mass of the substance necessary to contain exactly Avogadro's number of atoms
D) The volume of atoms in the substance divided by Avogadro's number
A) The amount of a substance that consists of as many entities as there are atoms in exactly 12 grams of the element carbon-12
B) The number of atoms of a substance found in 10 mL of the substance
C) The total mass of the substance necessary to contain exactly Avogadro's number of atoms
D) The volume of atoms in the substance divided by Avogadro's number

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following best describes the principle behind the unit conversion technique known as "dimensional analysis"?
A) All unit systems are able to use prefixes.
B) Any quantity can be multiplied by "1" without its value being changed.
C) Avogadro's number can identify the number of atoms in a mole.
D) The unit "mole" is standard in all unit systems.
A) All unit systems are able to use prefixes.
B) Any quantity can be multiplied by "1" without its value being changed.
C) Avogadro's number can identify the number of atoms in a mole.
D) The unit "mole" is standard in all unit systems.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following equations describes the relationship between the Kelvin scale and the Celsius scale?
A) K = °C + 273.15
B) K = (°C ×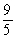 ) + 32
) + 32
C) K =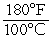
D) K = °C × 190
A) K = °C + 273.15
B) K = (°C ×
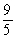 ) + 32
) + 32C) K =
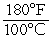
D) K = °C × 190

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
12
In the International System of Units (SI),what is the basic unit of measurement for temperature?
A) Celsius
B) Degrees
C) Fahrenheit
D) Kelvin
A) Celsius
B) Degrees
C) Fahrenheit
D) Kelvin

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
13
What is the first step in converting metric system units involving prefixes using Approach 1?
A) Determine the equivalence of the starting and target factors.
B) Locate the starting prefix and the target prefix and note their corresponding factors.
C) Locate the starting prefix and the target prefix and note their corresponding powers of 10.
D) Set up an equation of ratios and cross-multiply.
A) Determine the equivalence of the starting and target factors.
B) Locate the starting prefix and the target prefix and note their corresponding factors.
C) Locate the starting prefix and the target prefix and note their corresponding powers of 10.
D) Set up an equation of ratios and cross-multiply.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
14
After identifying the powers of 10 for the starting prefix and target prefix,what is the next step in converting metric system units using Approach 1?
A) Add the starting value to 10Δˣ, where Δx is the product of the powers of 10.
B) Divide the starting value by 10Δˣ, where Δx is the sum of the powers of 10.
C) Multiply the starting value by 10Δˣ, where Δx is the difference between the powers of 10.
D) Raise the starting value to the power of 10Δˣ, where Δx is the dividend of the powers of 10.
A) Add the starting value to 10Δˣ, where Δx is the product of the powers of 10.
B) Divide the starting value by 10Δˣ, where Δx is the sum of the powers of 10.
C) Multiply the starting value by 10Δˣ, where Δx is the difference between the powers of 10.
D) Raise the starting value to the power of 10Δˣ, where Δx is the dividend of the powers of 10.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
15
Avogadro's number is 602,213,700,000,000,000,000,000.What does this number represent?
A) The total number of atoms in 10 grams of a substance
B) The total number of moles in 10 grams of a substance
C) The total number of atoms in 12 grams of carbon-12
D) The total number of moles in 12 grams of carbon-12
A) The total number of atoms in 10 grams of a substance
B) The total number of moles in 10 grams of a substance
C) The total number of atoms in 12 grams of carbon-12
D) The total number of moles in 12 grams of carbon-12

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
16
At what temperature does water boil on the Fahrenheit scale?
A) 0°
B) 32°
C) 100°
D) 212°
A) 0°
B) 32°
C) 100°
D) 212°

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
17
A volume of fluid is measured as 2 pints.Under the United States Customary System of Units,how many quarts is this?
A) 1
B) 2
C) 4
D) 8
A) 1
B) 2
C) 4
D) 8

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
18
What is the formula weight of a substance?
A) The number of different substances in the chemical formula divided by Avogadro's number
B) The number of moles of each substance in a chemical formula divided by Avogadro's number
C) The product of the number of moles of each substance in the chemical formula
D) The sum of all the atomic weights of the chemical formula
A) The number of different substances in the chemical formula divided by Avogadro's number
B) The number of moles of each substance in a chemical formula divided by Avogadro's number
C) The product of the number of moles of each substance in the chemical formula
D) The sum of all the atomic weights of the chemical formula

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
19
How does expressing terms in the unit mole simplify calculations?
A) It deletes zeros.
B) It is easier to convert the value to liters.
C) It minimizes the number of necessary calculations.
D) It reduces the value of the measurement.
A) It deletes zeros.
B) It is easier to convert the value to liters.
C) It minimizes the number of necessary calculations.
D) It reduces the value of the measurement.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck
20
After identifying the factors of the starting prefix and target prefix,what is the next step in converting metric system units using Approach 2?
A) Calculate the ratio of the larger factor to the smaller factor.
B) Determine the sum of the larger factor and the smaller factor.
C) Multiply the starting value by 10Δˣ, where Δx is the difference between the powers of 10.
D) Set up an equation of ratios and subtract the larger ratio from the smaller ratio.
A) Calculate the ratio of the larger factor to the smaller factor.
B) Determine the sum of the larger factor and the smaller factor.
C) Multiply the starting value by 10Δˣ, where Δx is the difference between the powers of 10.
D) Set up an equation of ratios and subtract the larger ratio from the smaller ratio.

Unlock Deck
Unlock for access to all 20 flashcards in this deck.
Unlock Deck
k this deck


