Deck 18: Determination of Macromolecular Structure
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/60
Play
Full screen (f)
Deck 18: Determination of Macromolecular Structure
1
The resolution of an imaging technique must be matched to the size of the objects that are to be observed. If you were to map your classroom with a 1-meter (roughly 3.3 feet) resolution, which features of the room will you be able to see?
I. white board
II. table
III. chair
IV. pencil
V. eraser
A) I
B) II
C) III
D) I, II, and III
E) IV and V
I. white board
II. table
III. chair
IV. pencil
V. eraser
A) I
B) II
C) III
D) I, II, and III
E) IV and V
I, II, and III
2
The resolution of an imaging technique must be matched to the size of the objects that are to be observed. If you were to map your classroom with a 1-cm resolution, which features of the room will you be able to see?
A) eraser
B) table
C) chair
D) pencil
E) all of the above
A) eraser
B) table
C) chair
D) pencil
E) all of the above
all of the above
3
Determining the 3D molecular structure of an enzyme allows researchers to predict several structural features of the enzyme. Select all features that can be investigated, once the 3D structure of an enzyme is available:
I. active site
II. substrate binding site
III. molecular mass
IV. isoelectric point
A) I
B) II
C) I and II
D) III and IV
E) All of the above
I. active site
II. substrate binding site
III. molecular mass
IV. isoelectric point
A) I
B) II
C) I and II
D) III and IV
E) All of the above
I and II
4
The 3D molecular structures of biological molecules can be determined using the following techniques. Select all that apply:
I. light microscopy
II. electron microscopy
III. NMR
IV. x-ray crystallography
A) I and II
B) I, II and III
C) II, III, and IV
D) I and IV
E) All of the above
I. light microscopy
II. electron microscopy
III. NMR
IV. x-ray crystallography
A) I and II
B) I, II and III
C) II, III, and IV
D) I and IV
E) All of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements is true regarding the resolution of x-ray structures of proteins?
I. A resolution of 5 Å is needed to observe -helices as rods in an x-ray structure.
II. A resolution of 0.5 Å is needed to observe -sheets in an x-ray structure.
III. An x-ray structure of a protein at a resolution of 1.0 Å is considered to be high resolution.
IV. A resolution of 2.0 Å is needed to distinguish side chains of amino acids in an x-ray structure.
V. A resolution of 1.5 Å is needed to observe hydrogen atoms in an x-ray structure.
A) I and II
B) I, II, and III
C) II and V
D) I, III, IV, and V
E) None of the above
I. A resolution of 5 Å is needed to observe -helices as rods in an x-ray structure.
II. A resolution of 0.5 Å is needed to observe -sheets in an x-ray structure.
III. An x-ray structure of a protein at a resolution of 1.0 Å is considered to be high resolution.
IV. A resolution of 2.0 Å is needed to distinguish side chains of amino acids in an x-ray structure.
V. A resolution of 1.5 Å is needed to observe hydrogen atoms in an x-ray structure.
A) I and II
B) I, II, and III
C) II and V
D) I, III, IV, and V
E) None of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
6
Electron microscopes are usually installed in a vibration-proof room. Even small vibrations such as a truck passing outside can make acquiring data difficult. This is due to ____________.
I. interference
II. high resolution of the instrument
III. high sensitivity of the instrument
IV. temperature
A) I and II
B) II and III
C) III and IV
D) I and IV
E) All of the above
I. interference
II. high resolution of the instrument
III. high sensitivity of the instrument
IV. temperature
A) I and II
B) II and III
C) III and IV
D) I and IV
E) All of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
7
A convenient method for looking at tissues and cells to observe the organelles is _________.
A) x-ray crystallography
B) chromatography
C) light microscopy
D) electron microscopy
E) none of the above
A) x-ray crystallography
B) chromatography
C) light microscopy
D) electron microscopy
E) none of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
8
Electron microscopy and x-ray crystallography have a higher resolution than light microscopy due to the ___________ of the radiation used in the instrument.
A) shorter wavelength and lower energy
B) shorter wavelength and higher energy
C) longer wavelength and higher energy
D) longer wavelength and lower energy
A) shorter wavelength and lower energy
B) shorter wavelength and higher energy
C) longer wavelength and higher energy
D) longer wavelength and lower energy

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
9
The Protein Data Bank, PDB, is an international database of 3D molecular structures of biomolecules obtained by using _______________.
I. x-ray crystallography
II. light microscopy
III. scanning calorimetry
IV. NMR
V. electron microscopy
A) I, II, and III
B) I, III, and V
C) III, IV and V
D) II, III, and V
E) I, IV and V
I. x-ray crystallography
II. light microscopy
III. scanning calorimetry
IV. NMR
V. electron microscopy
A) I, II, and III
B) I, III, and V
C) III, IV and V
D) II, III, and V
E) I, IV and V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
10
A major difference between light microscopy and electron microscopy lies in the ______of light used and ________ of the two methods.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
11
To analyze a sample using an electron microscope, the sample must be _______ and observed in vacuum, due to _____________.
A) cleaned; safety
B) bleached; contamination
C) dried; the use of high-energy electrons
D) soaked; impurities
E) sonicated; safety
A) cleaned; safety
B) bleached; contamination
C) dried; the use of high-energy electrons
D) soaked; impurities
E) sonicated; safety

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
12
Electron microscopy images do not provide enough detail at the level of _______. Thus, data collected by electron microscopy cannot be validated using a Ramachandran plot to analyze the structure in terms of ___________ and _____________.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
13
Electron microscopes use several ____________ lenses to focus and direct the beam of electrons.
A) optical
B) electromagnetic
C) quartz
D) glass
E) transparent
A) optical
B) electromagnetic
C) quartz
D) glass
E) transparent

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
14
Stains used in electron microscopy such as phosphotungstenic acid and uranyl acetate are preferred over organic dyes because they ____________________, thus interact with the high-energy electrons more effectively.
A) are cheaper
B) have a brighter color
C) contain heavy atoms
D) bind better
E) are soluble
A) are cheaper
B) have a brighter color
C) contain heavy atoms
D) bind better
E) are soluble

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
15
The _____________ of a light microscope is 0.22 µm. This is due to the ____________ of light in the visible spectrum.
A) power; color
B) intensity; brightness
C) resolution; wavelength
D) contrast; speed
E) intensity; power
A) power; color
B) intensity; brightness
C) resolution; wavelength
D) contrast; speed
E) intensity; power

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
16
Contrast is ________________.
I. not important in imaging techniques
II. the ability of an object to stand out from its background
III. important in numerous imaging techniques, such as light microscopy and electron microscopy
IV. not used in facial recognition techniques
A) I and II
B) I, II, and III
C) II and III
D) I, III, and IV
I. not important in imaging techniques
II. the ability of an object to stand out from its background
III. important in numerous imaging techniques, such as light microscopy and electron microscopy
IV. not used in facial recognition techniques
A) I and II
B) I, II, and III
C) II and III
D) I, III, and IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
17
______________ is used to determine molecular structures using flash frozen samples and high-energy electrons.
A) Cryogenic electron microscopy (cryo-EM)
B) Light microscopy
C) Solution NMR
D) Transmission electron microscopy
E) MRI
A) Cryogenic electron microscopy (cryo-EM)
B) Light microscopy
C) Solution NMR
D) Transmission electron microscopy
E) MRI

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
18
Contrast agents used in electron microscopy are compounds of ____________________ that either bind to specific sites on the biomolecules or simply surround the biomolecules. (Note: choose all that apply)
A) heavy transitional metals
B) non-metals
C) metalloids
D) alkali metals
A) heavy transitional metals
B) non-metals
C) metalloids
D) alkali metals

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
19
Advances in electron microscopy and computer technology make it possible to improve the resolution of cryo-EM, such that large biomolecular assemblies can be structurally analyzed, providing information about ______.
I. protein - ligand interactions
II. multimeric protein assemblies
III. degradation mechanisms
IV. excessive interactions
V. weak associations
A) I and II
B) II and III
C) I and IV
D) III, IV and V
E) II and V
I. protein - ligand interactions
II. multimeric protein assemblies
III. degradation mechanisms
IV. excessive interactions
V. weak associations
A) I and II
B) II and III
C) I and IV
D) III, IV and V
E) II and V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
20
Developments of modern molecular biology and biochemistry techniques such as site-directed mutagenesis have led to an explosion of new structural data collected on a large variety of biomolecules, due to _____________________.
A) expression of proteins in bacteria
B) design of engineered mutations in scaffold proteins
C) design of enzymes
D) microdialysis techniques
E) all of the above
A) expression of proteins in bacteria
B) design of engineered mutations in scaffold proteins
C) design of enzymes
D) microdialysis techniques
E) all of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
21
______________ uses crystallized biomolecules in order to improve the signal of the diffraction pattern.
A) Crystallization
B) X-ray crystallography
C) NMR
D) light microscopy
E) Calorimetry
A) Crystallization
B) X-ray crystallography
C) NMR
D) light microscopy
E) Calorimetry

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
22
Order the steps that are needed to determine the 3D structure of a protein by x-ray crystallography.
I. diffraction pattern recorded using a diffractometer
II. data analysis
III. protein crystallization
IV. protein expression and purification
A) I, II, III, IV
B) IV, III, I, II
C) II, I, IV, III
D) III, I, II, IV
E) IV, I, II, III
I. diffraction pattern recorded using a diffractometer
II. data analysis
III. protein crystallization
IV. protein expression and purification
A) I, II, III, IV
B) IV, III, I, II
C) II, I, IV, III
D) III, I, II, IV
E) IV, I, II, III

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
23
In order to achieve high purity and molecular uniformity a purified protein sample has to be treated with ______and ______. Then, this protein can be used to grow single crystals for x-ray crystallography.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
24
Membrane proteins are usually difficult to crystallize and require the addition of _________ during purification and crystallization.
A) sucrose
B) octyl glucoside
C) lipids
D) sodium dodecylsulfate
E) none of the above
A) sucrose
B) octyl glucoside
C) lipids
D) sodium dodecylsulfate
E) none of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
25
Growing perfect single crystals of biological molecules is difficult and requires varying of the following parameters during crystallization:
A) temperature
B) pH
C) concentration of polyethylene glycol
D) concentration of glycerol
E) all of the above
A) temperature
B) pH
C) concentration of polyethylene glycol
D) concentration of glycerol
E) all of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
26
To achieve a high-quality single crystal of a protein, the solvent needs to be removed by _________.
I. boiling it off
II. slowly evaporating it
III. slowly diffusing it through a microdialysis membrane
IV. centrifuging it
A) I and II
B) II and III
C) I, III, and IV
D) II and IV
E) none of the above
I. boiling it off
II. slowly evaporating it
III. slowly diffusing it through a microdialysis membrane
IV. centrifuging it
A) I and II
B) II and III
C) I, III, and IV
D) II and IV
E) none of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
27
Choose all the methods that can be used to crystallize proteins and nucleic acids:
I. transformation
II. hanging drop crystallization
III. sitting drop crystallization
IV. diffraction
V. microdialysis
A) I and II
B) I, II, and III
C) II, III, and IV
D) III, IV, and V
E) I, III, and V
I. transformation
II. hanging drop crystallization
III. sitting drop crystallization
IV. diffraction
V. microdialysis
A) I and II
B) I, II, and III
C) II, III, and IV
D) III, IV, and V
E) I, III, and V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
28
High-quality protein crystals used in x-ray crystallography ____________.
I. can be as small as 0.1 mm
II. should have few impurities
III. cannot have cracks
IV. can be needle-shaped
V. can be oil-like
A) I and II
B) I, II and III
C) I, III, and IV
D) II, III and V
E) III, IV, and V
I. can be as small as 0.1 mm
II. should have few impurities
III. cannot have cracks
IV. can be needle-shaped
V. can be oil-like
A) I and II
B) I, II and III
C) I, III, and IV
D) II, III and V
E) III, IV, and V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
29
Some x-ray diffractometers use x-rays of various wavelengths that can be generated by ____________.
A) using a cyclotron or particle accelerator
B) a xenon lamp
C) a radioactive source
D) all of the above
E) none of the above
A) using a cyclotron or particle accelerator
B) a xenon lamp
C) a radioactive source
D) all of the above
E) none of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
30
Monochromatic x-rays used in x-ray diffractometers are generated by ________________.
A) a radioactive source
B) bombarding a metal surface with high-energy electrons
C) changing the metal
D) increasing the intensity
E) all of the above
A) a radioactive source
B) bombarding a metal surface with high-energy electrons
C) changing the metal
D) increasing the intensity
E) all of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
31
The advantages of using a synchrotron for x-ray crystallography are _____________________.
A) the x-rays are polychromatic
B) the x-rays are bright
C) data collection is fast
D) data has high resolution
E) all of the above
A) the x-rays are polychromatic
B) the x-rays are bright
C) data collection is fast
D) data has high resolution
E) all of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
32
X-ray diffraction data is used to generate the ___________ of the protein that can be used to build the 3D structure of the protein or nucleic acid.
A) map of the atoms
B) distribution of atoms
C) electron density map
D) grid of the bonds
E) none of the above
A) map of the atoms
B) distribution of atoms
C) electron density map
D) grid of the bonds
E) none of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
33
The ________________________ method is used to solve the _______________ in x-ray crystallography by comparing diffraction data before and after soaking the protein crystal in solutions made with heavy atoms, such as mercury, gold, or iodine.
A) multiple string replacement; phase problem
B) multiple isoelectric focusing; phase problem
C) multiple isomorphous replacement; phase solution
D) multiple isomorphous replacement; phase problem
E) none of the above
A) multiple string replacement; phase problem
B) multiple isoelectric focusing; phase problem
C) multiple isomorphous replacement; phase solution
D) multiple isomorphous replacement; phase problem
E) none of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
34
X-ray crystallography uses wavelengths of x-rays in the range of 0.1 - 1 Å which are similar to the ____.
I. resolution
II. size of atoms in the structure
III. structure
IV. lengths of covalent bonds
V. all of the above
A) I, II, and III
B) II, III, and IV
C) II and IV
D) III, IV and V
E) all of the above
I. resolution
II. size of atoms in the structure
III. structure
IV. lengths of covalent bonds
V. all of the above
A) I, II, and III
B) II, III, and IV
C) II and IV
D) III, IV and V
E) all of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
35
The root-mean-square deviation (RMSD) of bond lengths and bond angles of molecular structures are reported in PDB files to help researchers distinguish between higher and lower quality structures. Well defined structures have ____________________.
A) RMSD for bond lengths less than 0.1 Å and RMSD for bond angles of more than 1.0°
B) RMSD for bond lengths less than 0.01 Å and RMSD for bond angles of about 1.0°
C) RMSD for bond lengths less than 0.01 Å and RMSD for bond angles of about 2.0°
D) RMSD for bond lengths less than 1 Å and RMSD for bond angles of about 1.5°
E) none of the above
A) RMSD for bond lengths less than 0.1 Å and RMSD for bond angles of more than 1.0°
B) RMSD for bond lengths less than 0.01 Å and RMSD for bond angles of about 1.0°
C) RMSD for bond lengths less than 0.01 Å and RMSD for bond angles of about 2.0°
D) RMSD for bond lengths less than 1 Å and RMSD for bond angles of about 1.5°
E) none of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
36
High quality x-ray structures of proteins have ________________
I. a reliability factor, R, between 0.2 and 0.6. A lower value indicates a more reliable structure.
II. a reliability factor, R, between 2 and 6. A higher value indicates a more reliable structure.
III. a temperature factor B between 30 Å2 and 60 Å2. A lower value indicates greater confidence in the data.
IV. a temperature factor B between 3 Å2 and 6 Å2. A higher value indicates greater confidence in the data
A) I and II
B) II and III
C) II and IV
D) I and III
E) III and IV
I. a reliability factor, R, between 0.2 and 0.6. A lower value indicates a more reliable structure.
II. a reliability factor, R, between 2 and 6. A higher value indicates a more reliable structure.
III. a temperature factor B between 30 Å2 and 60 Å2. A lower value indicates greater confidence in the data.
IV. a temperature factor B between 3 Å2 and 6 Å2. A higher value indicates greater confidence in the data
A) I and II
B) II and III
C) II and IV
D) I and III
E) III and IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
37
The phi and psi bond angles in solved x-ray structures of proteins are mapped on a _______________ plot to verify that the secondary structure elements fall in the predicted areas.
A) protein
B) Southern
C) Scatchard
D) Ramachandran
E) Hill
A) protein
B) Southern
C) Scatchard
D) Ramachandran
E) Hill

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
38
The files of x-ray structures available in the Protein Data Bank, PDB, list a B factor, known as a temperature factor. This factor describes ___________________.
I. how large a biomolecule is
II. how strong the interactions between the chains are
III. how much flexibility various loops have in the structure
IV. how much thermal motion there was in the crystal in various regions
V. linearity of the molecule
A) I and II
B) II and III
C) III and IV
D) I and V
E) II and V
I. how large a biomolecule is
II. how strong the interactions between the chains are
III. how much flexibility various loops have in the structure
IV. how much thermal motion there was in the crystal in various regions
V. linearity of the molecule
A) I and II
B) II and III
C) III and IV
D) I and V
E) II and V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
39
The 3D structures of biomolecules are available in the Protein Data Bank, PDB. They were determined by using ______.
A) x-ray crystallography
B) electron microscopy
C) NMR
D) neutron scattering
E) all of the above
A) x-ray crystallography
B) electron microscopy
C) NMR
D) neutron scattering
E) all of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
40
X-rays interact with the electron clouds in the structures of proteins, while neutrons used in neutron scattering interact with the ___________________.
A) electron orbitals
B) covalent bonds
C) space
D) nuclei of the atoms
E) electron spins
A) electron orbitals
B) covalent bonds
C) space
D) nuclei of the atoms
E) electron spins

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
41
Statistical data on the experimental setup of an x-ray diffraction structure includes _________________.
I. total number of reflections recorded
II. number of atoms
III. types of heavy atoms used in phasing the crystal
IV. number of residues
V. shape and dimension of the unit cell
A) I, II, and III
B) II, III and IV
C) I, IV, and V
D) II, III and IV.
E) I, III and V
I. total number of reflections recorded
II. number of atoms
III. types of heavy atoms used in phasing the crystal
IV. number of residues
V. shape and dimension of the unit cell
A) I, II, and III
B) II, III and IV
C) I, IV, and V
D) II, III and IV.
E) I, III and V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
42
Look at the data presented in the image and choose which statements are true:
I. The data show a well-resolved x-ray structure, due to the low RMDS values and acceptable B and Rwork values.
II. The data show a poorly-solved x-ray structure due to the high RMDS values and out of range B and Rwork values.
III. The data were recorded at a good resolution, in the range 2.7 - 3.1 Å.
IV. The data show that the x-ray structure does not contain any solvent molecules.
V. The data show a poorly-solved x-ray structure due to the high number of atoms.
A) I, II, and III
B) I, III and IV
C) II, IV and V
D) III, IV and V
E) I, III and V
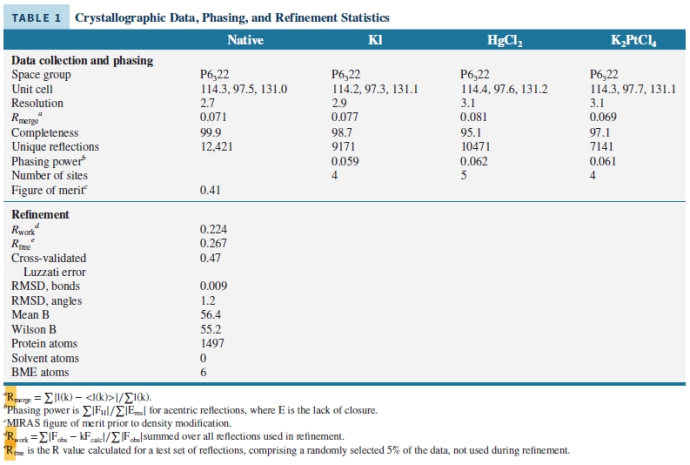
I. The data show a well-resolved x-ray structure, due to the low RMDS values and acceptable B and Rwork values.
II. The data show a poorly-solved x-ray structure due to the high RMDS values and out of range B and Rwork values.
III. The data were recorded at a good resolution, in the range 2.7 - 3.1 Å.
IV. The data show that the x-ray structure does not contain any solvent molecules.
V. The data show a poorly-solved x-ray structure due to the high number of atoms.
A) I, II, and III
B) I, III and IV
C) II, IV and V
D) III, IV and V
E) I, III and V


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
43
The difficulty in using x-ray crystallography to determine the 3D structure of a protein relies in __________.
I. solving the diffraction data
II. transferring data
III. growing single crystals
IV. solving the phase problem by multiple isomorphous replacement
V. stabilizing the crystal
A) I and II
B) I and III
C) II, III, and V
D) III and IV
E) I, III and V
I. solving the diffraction data
II. transferring data
III. growing single crystals
IV. solving the phase problem by multiple isomorphous replacement
V. stabilizing the crystal
A) I and II
B) I and III
C) II, III, and V
D) III and IV
E) I, III and V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
44
Neutrons interact stronger with _____________ atoms than x-rays.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
45
Which statement is true about nuclear magnetic resonance, NMR?
A) NMR is used in organic chemistry to determine the structure of organic molecules.
B) NMR is used in biochemistry to determine the structure of biomolecules in solution.
C) NMR is used in a clinical setting as a diagnostic tool, called MRI.
D) NMR is a non-destructive analytical technique
E) all of the above
A) NMR is used in organic chemistry to determine the structure of organic molecules.
B) NMR is used in biochemistry to determine the structure of biomolecules in solution.
C) NMR is used in a clinical setting as a diagnostic tool, called MRI.
D) NMR is a non-destructive analytical technique
E) all of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
46
The following nuclei are all spin active and are used in NMR experiments. The ones that have a ___________ abundance are ___(list all)_______ and they are more useful in biochemical experiments.
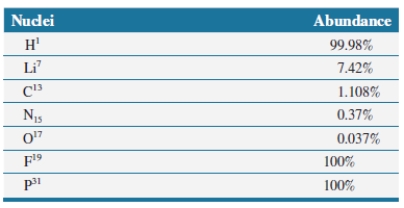


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
47
The pharmaceutical industry uses F19-NMR to characterize novel drug compounds. This is possible only if those drugs ______.
I. are soluble
II. contain fluorine in their molecule
III. are easy to synthesize
IV. can ionize
A) I and II
B) II and III
C) III and IV
D) I and III
E) II and IV
I. are soluble
II. contain fluorine in their molecule
III. are easy to synthesize
IV. can ionize
A) I and II
B) II and III
C) III and IV
D) I and III
E) II and IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
48
A high-field NMR instrument uses a _______________.
A) superconducting cryomagnet cooled with liquid helium or nitrogen
B) radiofrequency pulse generator
C) probe
D) detector
E) all of the above
A) superconducting cryomagnet cooled with liquid helium or nitrogen
B) radiofrequency pulse generator
C) probe
D) detector
E) all of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
49
The result of an NMR experiment is a __________. Often tetramethylsilane (TMS) is used as a ___________ peak in an H-NMR experiment.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
50
The chemical shift, , is independent of the NMR instrument used, since it is defined as a ratio of frequencies. Choose all statements that are true regarding the chemical shift, .
I. The chemical shift, , is a characteristic of the electronic environment that the atoms are in.
II. The chemical shift of the H in tetramethylsilane (TMS) is set 0 ppm as a reference.
III. Compounds containing a single type of hydrogen atom will show only one peak in the H-NMR spectrum.
IV. The chemical shift, is increasing left to right on an NMR spectrum.
A) I and II
B) I, II and III
C) II and IV
D) III and IV
E) All of the above
I. The chemical shift, , is a characteristic of the electronic environment that the atoms are in.
II. The chemical shift of the H in tetramethylsilane (TMS) is set 0 ppm as a reference.
III. Compounds containing a single type of hydrogen atom will show only one peak in the H-NMR spectrum.
IV. The chemical shift, is increasing left to right on an NMR spectrum.
A) I and II
B) I, II and III
C) II and IV
D) III and IV
E) All of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
51
One-dimensional NMR spectra are analyzed based on the _____________.
A) number of peaks
B) position of the peaks relative to a standard
C) splitting of the peaks
D) number of equivalent nuclei in each peak, calculated by integrating the area under the peak
E) all of the above
A) number of peaks
B) position of the peaks relative to a standard
C) splitting of the peaks
D) number of equivalent nuclei in each peak, calculated by integrating the area under the peak
E) all of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
52
NMR structures in the PDB do not list the reliability factor, R, and the temperature factor, B, as is customary for x-ray structures. This is due to the fact that _____________________.
I. NMR structures have a lower reliability
II. temperature does not affect NMR structures
III. NMR does not use diffraction data and electron density maps
IV. NMR uses chemical shift correlations
A) I and II
B) I and III
C) III and IV
D) II and III
E) I and IV
I. NMR structures have a lower reliability
II. temperature does not affect NMR structures
III. NMR does not use diffraction data and electron density maps
IV. NMR uses chemical shift correlations
A) I and II
B) I and III
C) III and IV
D) II and III
E) I and IV

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
53
_________________ generates the highest resolution 3D biomolecular structures.
A) Cryoelectron microscopy
B) Light microscopy
C) X-ray crystallography
D) NMR
E) Calorimetry
A) Cryoelectron microscopy
B) Light microscopy
C) X-ray crystallography
D) NMR
E) Calorimetry

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
54
During multidimensional NMR the sample is pulsed with a radio-frequency at different angles, which leads to _________. Choose all that apply.
I. different relaxation times
II. heating of the sample
III. identification of nuclei which are within three bonds of each other in the molecule
IV. identification of nuclei which are spatially in close-proximity in the folded protein
V. identification of side chains
A) I, II and III
B) II, III and V
C) II, III and IV
D) I, III and IV
E) II and V
I. different relaxation times
II. heating of the sample
III. identification of nuclei which are within three bonds of each other in the molecule
IV. identification of nuclei which are spatially in close-proximity in the folded protein
V. identification of side chains
A) I, II and III
B) II, III and V
C) II, III and IV
D) I, III and IV
E) II and V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
55
The ______________ equation is used in interpreting protein NMR data.
A) Wetterhahn
B) Sanger
C) Djerassi
D) Karplus
E) Wüthrich
A) Wetterhahn
B) Sanger
C) Djerassi
D) Karplus
E) Wüthrich

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
56
NMR can be used for determining the 3D structure of proteins in solution. This allows for getting insight into how the protein structure ________________.
A) changes when temperature changes
B) looks like in its biologically active form
C) changes when the ionic strength of the solution changes
D) accommodates the binding of ligands
E) all of the above
A) changes when temperature changes
B) looks like in its biologically active form
C) changes when the ionic strength of the solution changes
D) accommodates the binding of ligands
E) all of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
57
Due to the complexity of biological molecules, NMR spectrometers that are used for analysis of biological molecules require a static field of _______.
A) 60 MHz
B) 100 MHz
C) at least 300 MHz
D) 500 MHz
E) 900 MHz
A) 60 MHz
B) 100 MHz
C) at least 300 MHz
D) 500 MHz
E) 900 MHz

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
58
Due to the complexity of biomolecules, multidimensional NMR techniques, such as _________ are needed to determine their 3D structure. Choose all that apply.
I. correlation spectroscopy (COSY)
II. electron energy loss spectroscopy (EELS)
III. total correlation spectroscopy (TOCSY)
IV. nuclear Overhauser effect spectroscopy (NOESY)
V. total electron yield (TEY)
A) I, II and III
B) II, III, and IV
C) I, III and IV
D) II, IV and V
E) All of the above
I. correlation spectroscopy (COSY)
II. electron energy loss spectroscopy (EELS)
III. total correlation spectroscopy (TOCSY)
IV. nuclear Overhauser effect spectroscopy (NOESY)
V. total electron yield (TEY)
A) I, II and III
B) II, III, and IV
C) I, III and IV
D) II, IV and V
E) All of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
59
Electron microscopy, x-ray crystallography and NMR spectroscopy are used to determine 3D structures of biomolecules. Using protein examples studied in chapter 3, which statements are true?
A) The structure of insulin can easily be studied in solution using NMR, since insulin has a small molecular weight.
B) The structure of immunoglobulin can be determined by electron microscopy.
can be determined by electron microscopy.
C) Fragments of immunoglobulin 11ef2e34_c901_7ebf_a6da_5387e8b94d05_TB9579_11 can be determined by x-ray crystallography.
D) X-ray crystallography is well-suited for determining the 3D structure of hemoglobin.
E) all of the above
A) The structure of insulin can easily be studied in solution using NMR, since insulin has a small molecular weight.
B) The structure of immunoglobulin
 can be determined by electron microscopy.
can be determined by electron microscopy.C) Fragments of immunoglobulin 11ef2e34_c901_7ebf_a6da_5387e8b94d05_TB9579_11 can be determined by x-ray crystallography.
D) X-ray crystallography is well-suited for determining the 3D structure of hemoglobin.
E) all of the above

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
60
Biochemical research is usually done by large research groups. The members have a variety of responsibilities and contributions to the project. Read the section Giving credit where is due in this chapter. Choose the sentences which best describe a fair way of listing authors on the research paper published by a particular research group at a university.
I. The first author on the paper is the one who has done most of the work on the study and the principal investigator (lab director or professor) is the last author.
II. Group members who contributed to some sections of the paper are listed.
III. The animal technician will be added as an author to some of the papers published by the group.
IV. Students will be listed as authors even if they have not contributed to this particular research.
V. The order of listing the names of the authors is not important.
A) I, II, and III
B) II, III, and IV
C) II, III and V
D) I, II and V
E) III, IV and V
I. The first author on the paper is the one who has done most of the work on the study and the principal investigator (lab director or professor) is the last author.
II. Group members who contributed to some sections of the paper are listed.
III. The animal technician will be added as an author to some of the papers published by the group.
IV. Students will be listed as authors even if they have not contributed to this particular research.
V. The order of listing the names of the authors is not important.
A) I, II, and III
B) II, III, and IV
C) II, III and V
D) I, II and V
E) III, IV and V

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck


