Deck 1: The Chemical Foundations of Biochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/28
Play
Full screen (f)
Deck 1: The Chemical Foundations of Biochemistry
1
If a reaction at 37 C has a H of 23 kJ/mol and a S of 337 J/K.mol, what is the G for the reaction?
A) 65 kJ/mol
B) -42 kJ/mol
C) 18 kJ/mol
D) -19 kJ/mol
E) none of the above
A) 65 kJ/mol
B) -42 kJ/mol
C) 18 kJ/mol
D) -19 kJ/mol
E) none of the above
-19 kJ/mol
2
Consider a reaction in which H = -20. kJ/mol and S = 10. J/mol · K.
a. G = H - S G = -20,000 J/mol - (298 × 10 J/mol) = -23,000 J/mol
b. The reaction is spontaneous because the G is negative.
b. The reaction is spontaneous because the G is negative.
3
Of the following amino acids, which contains an alcohol? 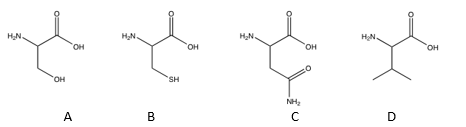
A) A
B) B
C) C
D) D
E) all of the above

A) A
B) B
C) C
D) D
E) all of the above
A
4
What functional groups are present in the following molecule? 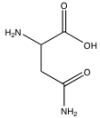
A) amine and carboxylic acid
B) amine, ketone, and carboxylic acid
C) amine, amide, and carboxylic acid
D) alcohol, amine, amide, and carboxylic acid
E) none of the above are correct

A) amine and carboxylic acid
B) amine, ketone, and carboxylic acid
C) amine, amide, and carboxylic acid
D) alcohol, amine, amide, and carboxylic acid
E) none of the above are correct

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
5
Keq can be determined from the change in standard free energy using the equation ______.
A) Keq = e- G°/RT
B) Keq = ln e- G°/T S
C) Keq = e- H/RT
D) Keq = e- G°/T S
E) Keq = log e- G°/RT
A) Keq = e- G°/RT
B) Keq = ln e- G°/T S
C) Keq = e- H/RT
D) Keq = e- G°/T S
E) Keq = log e- G°/RT

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
6
Consider the isomerization reaction R P, in which R is converted to P. The G°' for this reaction is -10. kJ/mol. Calculate the [P]/[R] at equilibrium at 25°C.

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
7
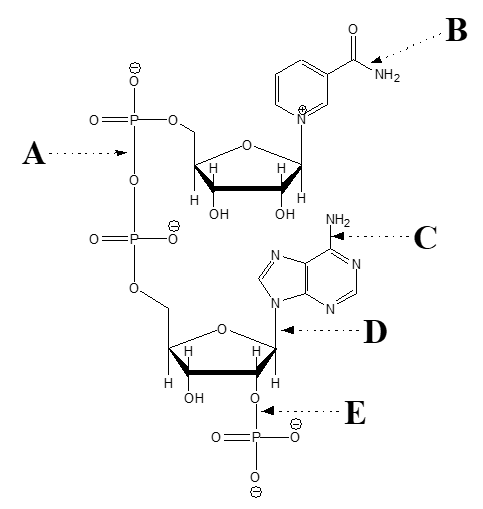
-Which arrow points at a phosphoester bond?
A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
8

-Which arrow points at an amide bond?
A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
9

-Which arrow points at a phosphoanhydride bond?
A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
10

-In a water molecule, hydrogens are partially _____; oxygens are partially _____.
A) negative; negative
B) negative; positive
C) positive; positive
D) positive; negative
E) none of the above

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
11
Due to the formation of hydrogen bonds, _____ is highly soluble in water.
A) carbon dioxide
B) sodium chloride
C) methanol
D) octane
E) cholesterol
A) carbon dioxide
B) sodium chloride
C) methanol
D) octane
E) cholesterol

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following explains the interactions that occur between the atoms of water molecules and the ions that form when sodium chloride dissolves in water?
A) Hydrogens interact with the sodium ion; oxygens interact with the chloride ion.
B) Hydrogens interact with the chloride ion; oxygens interact with the sodium ion.
C) Hydrogens interact with the sodium ion and the chloride ion.
D) Oxygens interact with the sodium ion and the chloride ion.
E) none of the above
A) Hydrogens interact with the sodium ion; oxygens interact with the chloride ion.
B) Hydrogens interact with the chloride ion; oxygens interact with the sodium ion.
C) Hydrogens interact with the sodium ion and the chloride ion.
D) Oxygens interact with the sodium ion and the chloride ion.
E) none of the above

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
13
The polarity of the O-H bond is caused by the ______ of oxygen relative to that of hydrogen.

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is a physical property of water that results from hydrogen bonding?
A) a high boiling point relative to molecular weight
B) a solid state that is less dense than the liquid state
C) high surface tension
D) the ability to solubilize polar molecules
E) all of the above
A) a high boiling point relative to molecular weight
B) a solid state that is less dense than the liquid state
C) high surface tension
D) the ability to solubilize polar molecules
E) all of the above

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following functional groups has two hydrogen bond donors and one hydrogen bond acceptor?
A) alcohol
B) ester
C) thiol
D) amine
E) amide
A) alcohol
B) ester
C) thiol
D) amine
E) amide

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
16
Urea is a water-soluble product of nitrogen metabolism. How many hydrogen bonds can one urea molecule donate to surrounding water molecules? 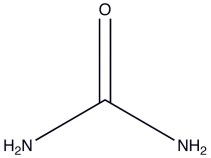
A) 2
B) 3
C) 4
D) 5
E) 6

A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
17
In an aqueous solution, if the [OH-] is 3.0 × 10-5 M, what is the [H+]?
A) 7.0 × 10-9
B) 7.0 × 10-2
C) 3.3 × 10-3
D) 3.3 × 10-10
E) none of the above
A) 7.0 × 10-9
B) 7.0 × 10-2
C) 3.3 × 10-3
D) 3.3 × 10-10
E) none of the above

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
18
What is the [H+] of an aqueous solution with a pH of 6.2?
A) 6.2 × 10-6
B) 1.6 × 10-8
C) 6.3 × 10-7
D) 3. 3 × 10-5
E) none of the above
A) 6.2 × 10-6
B) 1.6 × 10-8
C) 6.3 × 10-7
D) 3. 3 × 10-5
E) none of the above

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
19
What would be the resulting pH if one drop (0.05 ml) of 1.0 M HCl was added to one liter of pure water (assume pH 7.0)?
A) 2.7
B) 4.3
C) 5.0
D) 7.0 (there would be no significant change)
E) 9.7
A) 2.7
B) 4.3
C) 5.0
D) 7.0 (there would be no significant change)
E) 9.7

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
20
What would be the resulting pH if one ml of 1.0 M NaOH was added to one liter of pure water (assume pH 7.0)?
A) 1
B) 3
C) 7.3
D) 11
E) 13
A) 1
B) 3
C) 7.3
D) 11
E) 13

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following would be the strongest acid?
A) formic acid, pK = 3.75
B) succinic acid, a diprotic acid with pK = 4.21 and 5.64
C) acetic acid, pK = 4.76
D) ammonium ion, pK = 9.25
E) cannot be determined from the given information
A) formic acid, pK = 3.75
B) succinic acid, a diprotic acid with pK = 4.21 and 5.64
C) acetic acid, pK = 4.76
D) ammonium ion, pK = 9.25
E) cannot be determined from the given information

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
22
What is the pH of a solution that contains three parts acetic acid and one part sodium acetate? The pK for acetic acid is 4.76.
A) 5.24
B) 5.06
C) 4.46
D) 4.28
E) cannot be determined from the given information
A) 5.24
B) 5.06
C) 4.46
D) 4.28
E) cannot be determined from the given information

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
23
If the pK values for phosphoric acid are 2.15, 6.82, and 12.38, at what pH would one observe equal amounts of H2PO4- and HPO42-?
A) 2.15
B) 4.49
C) 6.82
D) 9.60
E) 12.38
A) 2.15
B) 4.49
C) 6.82
D) 9.60
E) 12.38

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
24
If the pK values for phosphoric acid are 2.15, 6.82 and 12.38, _____ would predominate at pH 5, and _____ would predominate at pH 10.
A) H3PO4; H2PO4-
B) H3PO4; HPO42-
C) H3PO4; PO43-
D) H2PO4-; PO43-
E) H2PO4-; HPO42-
A) H3PO4; H2PO4-
B) H3PO4; HPO42-
C) H3PO4; PO43-
D) H2PO4-; PO43-
E) H2PO4-; HPO42-

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
25
What is the conjugate acid of H2PO4-?
A) HPO42-
B) H2PO4
C) H3PO4
D) PO43-
E) none of the above
A) HPO42-
B) H2PO4
C) H3PO4
D) PO43-
E) none of the above

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following shows the buffer that is found in the bloodstream?
A) H3PO4 H2PO42- + H+
B) H2PO4- HPO42- + H+
C) HPO42- PO43- + H+
D) H2CO3 HCO3- + H+
E) HCO3- CO32- + H+
A) H3PO4 H2PO42- + H+
B) H2PO4- HPO42- + H+
C) HPO42- PO43- + H+
D) H2CO3 HCO3- + H+
E) HCO3- CO32- + H+

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
27
If a phosphate buffer (pK = 6.82) was formulated such that its pH was 7.3, it would be best suited to buffer against _____. If instead, it was formulated such that its pH was 6.3, it would be best suited to buffer against _____.
A) acid; base
B) acid; acid
C) base; acid
D) base; base
E) a buffer with a pH that far from the pK would not be an effective buffer
A) acid; base
B) acid; acid
C) base; acid
D) base; base
E) a buffer with a pH that far from the pK would not be an effective buffer

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck
28
Metabolic acidosis often causes increased respiratory rates. What portion of the bloodstream buffer is lost through increased respiration?
A) H+
B) HCO3-
C) H2CO3
D) CO2
E) H2O
A) H+
B) HCO3-
C) H2CO3
D) CO2
E) H2O

Unlock Deck
Unlock for access to all 28 flashcards in this deck.
Unlock Deck
k this deck


