Deck 40: Nuclei
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/46
Play
Full screen (f)
Deck 40: Nuclei
1
Acceptable dimensions of the mass number A are
A) kilograms.
B) joules.
C) either of the first two responses, because
D) neither of the first two responses is correct.
A) kilograms.
B) joules.
C) either of the first two responses, because
D) neither of the first two responses is correct.
neither of the first two responses is correct.
2
The nuclear radius of 64Co is approximately
A) 1.2 fm.
B) 4.8 fm.
C) 6.0 fm.
D) 12 fm.
E) None of the above responses is correct.
A) 1.2 fm.
B) 4.8 fm.
C) 6.0 fm.
D) 12 fm.
E) None of the above responses is correct.
12 fm.
3
The nuclear radius of 27Co is approximately
A) 1.2 fm.
B) 3.6 fm.
C) 4.5 fm.
D) 6.0 fm.
E) None of the above responses is correct.
A) 1.2 fm.
B) 3.6 fm.
C) 4.5 fm.
D) 6.0 fm.
E) None of the above responses is correct.
3.6 fm.
4
The density of nuclear matter is of the order of
A) 103 kg/m3.
B) 107 kg/m3.
C) 1017 kg/m3.
D) 1050 kg/m3.
E) None of the above responses is correct.
A) 103 kg/m3.
B) 107 kg/m3.
C) 1017 kg/m3.
D) 1050 kg/m3.
E) None of the above responses is correct.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
5
A valid inference from the fact that the radius of the nucleus is related to the mass number A is that
A) the nuclear force obeys an inverse square law.
B) the number of nucleons per unit volume in atoms is independent of the element.
C) the atomic size increases as the nuclear size increases.
D) the nucleons in a nucleus are separated by distances large compared to their individual sizes.
A) the nuclear force obeys an inverse square law.
B) the number of nucleons per unit volume in atoms is independent of the element.
C) the atomic size increases as the nuclear size increases.
D) the nucleons in a nucleus are separated by distances large compared to their individual sizes.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
6
Compared to atomic energies, nuclear energies are
A) a million times larger.
B) a thousand times larger.
C) of about the same magnitude.
D) a thousand times smaller.
E) a million times smaller.
A) a million times larger.
B) a thousand times larger.
C) of about the same magnitude.
D) a thousand times smaller.
E) a million times smaller.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
7
All isotones of a given element
A) have the same chemical properties.
B) have the same number of protons.
C) have the same number of nucleons.
D) have the same number of neutrons.
E) All of the above are true.
A) have the same chemical properties.
B) have the same number of protons.
C) have the same number of nucleons.
D) have the same number of neutrons.
E) All of the above are true.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
8
The graphs here show the number of protons as a function of the number of neutrons. Two nuclides that are isotopes could lie on the curve in graph
A)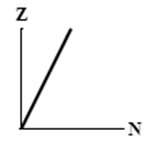
B)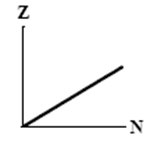
C)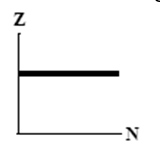
D)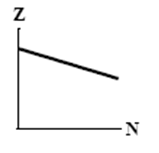
E)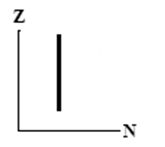
A)
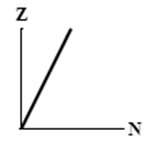
B)

C)
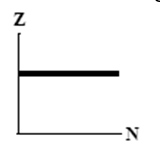
D)

E)
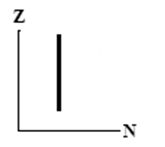

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
9
The graphs here show the number of protons as a function of the number of neutrons. Two nuclides that are isotones could lie on the curve in graph
A)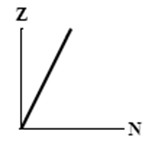
B)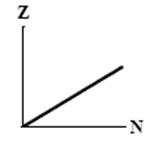
C)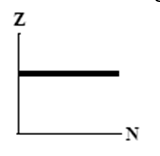
D)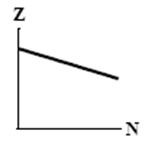
E)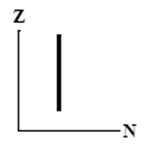
A)
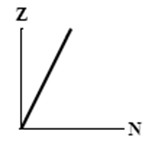
B)

C)
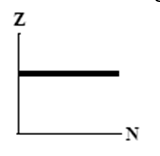
D)

E)
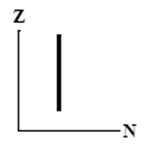

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
10
In comparison with an electron, a proton has larger
A) mass and size.
B) spin.
C) magnetic moment.
D) All of the previous answers are correct.
E) None of the previous answers is correct.
A) mass and size.
B) spin.
C) magnetic moment.
D) All of the previous answers are correct.
E) None of the previous answers is correct.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
11
In comparison with an electron, a proton has the same
A) mass and size.
B) spin.
C) magnetic moment.
D) All of the previous answers are correct.
E) None of the previous answers is correct.
A) mass and size.
B) spin.
C) magnetic moment.
D) All of the previous answers are correct.
E) None of the previous answers is correct.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
12
In a nucleus, the numbers of protons, neutrons, and electrons are
A) 56, 79, and 135, respectively.
B) 56, 79, and 0, respectively.
C) 79, 56, and 135, respectively.
D) 79, 56, and 0, respectively.
E) None of the previous answers is correct.
A) 56, 79, and 135, respectively.
B) 56, 79, and 0, respectively.
C) 79, 56, and 135, respectively.
D) 79, 56, and 0, respectively.
E) None of the previous answers is correct.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
13
Of the total nuclear rest-mass energy, the nuclear binding energy amounts to approximately
A) 0.1%.
B) 1.0%.
C) 10%.
D) 50%.
E) 90%.
A) 0.1%.
B) 1.0%.
C) 10%.
D) 50%.
E) 90%.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
14
The mass of a typical nucleus is
A) somewhat less than the sum of the masses of its constituents.
B) somewhat more than the sum of the masses of its constituents.
C) equal to the sum of the masses of its constituents.
D) None of the previous responses is a valid generalization.
A) somewhat less than the sum of the masses of its constituents.
B) somewhat more than the sum of the masses of its constituents.
C) equal to the sum of the masses of its constituents.
D) None of the previous responses is a valid generalization.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
15
In terms of increasing penetrating ability, the three kinds of rays emitted by radioactive isotopes are
A)
B)
C)
D)
E) All have the same penetrating ability.
A)
B)
C)
D)
E) All have the same penetrating ability.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
16
In terms of charge, the rays emitted by radioactive isotopes are, respectively
A) positive, neutral, and negative.
B) positive, negative, and neutral.
C) negative, neutral, and positive.
D) negative, positive, and neutral.
E) neutral, negative, and positive.
A) positive, neutral, and negative.
B) positive, negative, and neutral.
C) negative, neutral, and positive.
D) negative, positive, and neutral.
E) neutral, negative, and positive.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
17
During decay,
A) a neutron is ejected from the nucleus.
B) a neutron is transformed to a proton.
C) a proton is transformed to a neutron.
D) a proton is ejected from the nucleus.
E) None of the above is true.
A) a neutron is ejected from the nucleus.
B) a neutron is transformed to a proton.
C) a proton is transformed to a neutron.
D) a proton is ejected from the nucleus.
E) None of the above is true.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
18
If the parent nucleus has N neutrons and Z protons, after an decay, the daughter nucleus has respective neutron and proton numbers of
A) N and Z - 1.
B) N and Z + 1.
C) N - 2 and Z - 2.
D) N - 1 and Z - 1.
E) N and Z.
A) N and Z - 1.
B) N and Z + 1.
C) N - 2 and Z - 2.
D) N - 1 and Z - 1.
E) N and Z.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
19
If the parent nucleus has N neutrons and Z protons, after the ejection of a positron (anti-electron) in a decay, the daughter nucleus has respective neutron and proton numbers of
A) N - 1 and Z - 1.
B) N - 1 and Z + 1.
C) N and Z - 2.
D) N + 1 and Z + 1.
E) N + 1 and Z - 1.
A) N - 1 and Z - 1.
B) N - 1 and Z + 1.
C) N and Z - 2.
D) N + 1 and Z + 1.
E) N + 1 and Z - 1.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
20
The radioactive decay mode where the daughter and the parent nuclides are the same element is
A) decay.
B) decay.
C) decay.
D) None of the previous responses is valid.
A) decay.
B) decay.
C) decay.
D) None of the previous responses is valid.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
21
When 238U emits an -particle and two -rays,
A) 234U is produced.
B) 238Th is produced.
C) 234Th is produced.
D) 236Th is produced.
E) 242Pu is produced.
A) 234U is produced.
B) 238Th is produced.
C) 234Th is produced.
D) 236Th is produced.
E) 242Pu is produced.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following is most likely to undergo fission?
A) 2H.
B) 4He.
C) 32P.
D) 56Fe.
E) 235U.
A) 2H.
B) 4He.
C) 32P.
D) 56Fe.
E) 235U.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is most likely to release energy when undergoing fusion with other nuclei?
A) 2H.
B) 4He.
C) 32P.
D) 56Fe.
E) 235U.
A) 2H.
B) 4He.
C) 32P.
D) 56Fe.
E) 235U.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
24
The binding energy per nucleon is greatest for nuclei of
A) small mass.
B) intermediate mass.
C) large mass.
D) More information is needed to work out the answer.
A) small mass.
B) intermediate mass.
C) large mass.
D) More information is needed to work out the answer.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
25
In decay it is found that both the direction of the ejected electron (with respect to the recoil direction of the parent nucleus) and the magnitude of the electron's velocity are variable. From these facts, we can infer that another particle (the neutrino, as it turns out) is needed to preserve the conservation of
A) energy.
B) momentum.
C) all of the above.
D) none of the above.
A) energy.
B) momentum.
C) all of the above.
D) none of the above.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
26
The significant forces in determining the structure of nuclei are
A) coulomb and gravitational.
B) gravitational and weak.
C) strong and weak.
D) coulomb and strong.
E) gravitational, coulomb, and strong.
A) coulomb and gravitational.
B) gravitational and weak.
C) strong and weak.
D) coulomb and strong.
E) gravitational, coulomb, and strong.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
27
The binding energy per nucleon is
A) the same for all atoms.
B) proportional to atomic number.
C) inversely proportional to atomic number.
D) strongly dependent on charge.
E) none of the above.
A) the same for all atoms.
B) proportional to atomic number.
C) inversely proportional to atomic number.
D) strongly dependent on charge.
E) none of the above.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
28
The atomic mass of the deuteron (the nucleus of the deuterium atom), which consists of a proton (mass 1.007825 u) and a neutron (mass 1.007825 u), is 2.014102 u. The binding energy of the deuteron is
A) 2.224 J.
B) 2.224 keV.
C) 2.224 MeV.
D) 3.754 keV.
E) 3.754 MeV.
A) 2.224 J.
B) 2.224 keV.
C) 2.224 MeV.
D) 3.754 keV.
E) 3.754 MeV.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
29
The nucleus with the larger binding energy per nucleon is
A) 238U.
B) 197Au.
C) 62Ni.
D) 4He.
E) 2H.
A) 238U.
B) 197Au.
C) 62Ni.
D) 4He.
E) 2H.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
30
The half-life of carbon-14 is about 6000 years. If the amount of carbon-14 remaining in a sample is 1/100 of the original amount, the atoms of the sample began decaying about
A) 20,000 years earlier.
B) 40,000 years earlier.
C) 60,000 years earlier.
D) 80,000 years earlier.
A) 20,000 years earlier.
B) 40,000 years earlier.
C) 60,000 years earlier.
D) 80,000 years earlier.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
31
If the half-life of a material is 45 years, how much will be left after 100 years?
A) less than a quarter.
B) more than a quarter.
C) less than half.
D) more than half.
E) More information is needed to work out the answer.
A) less than a quarter.
B) more than a quarter.
C) less than half.
D) more than half.
E) More information is needed to work out the answer.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
32
As a radioactive substance decays, its half-life
A) increases.
B) stays the same.
C) decreases.
A) increases.
B) stays the same.
C) decreases.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
33
A sample of 131I (which has a half-life of 8.04 days) has an activity of 5.0 mCi at the time of shipment, and an activity of 4.2 mCi upon receipt in a medical laboratory. The time elapsed between the shipment and the receipt is
A) 6.8 days.
B) 6.4 days.
C) 5.0 days.
D) 2.5 days.
E) 2.0 days.
A) 6.8 days.
B) 6.4 days.
C) 5.0 days.
D) 2.5 days.
E) 2.0 days.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
34
The number of 131I (half-life of 8.04 days) nuclei necessary to produce an activity of 5.0 mCi is
A) 8.91 * 1014.
B) 1.85 * 1014.
C) 3.09 * 1012.
D) 5.01 * 106.
E) 5.01 * 103.
A) 8.91 * 1014.
B) 1.85 * 1014.
C) 3.09 * 1012.
D) 5.01 * 106.
E) 5.01 * 103.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
35
A 300-g sample of 218Po undergoes decay with a half-life of 3.1 minutes. After 15.5 minutes,
A) 9.38 g of 214Pb would be formed.
B) 18.8 g of 214Pb would be formed.
C) 120 g of 214Pb would be formed.
D) 281 g of 214Pb would be formed.
E) 291 g of 214Pb would be formed.
A) 9.38 g of 214Pb would be formed.
B) 18.8 g of 214Pb would be formed.
C) 120 g of 214Pb would be formed.
D) 281 g of 214Pb would be formed.
E) 291 g of 214Pb would be formed.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
36
The radioactivity of a certain radioisotope decreases to one-half in 1.0 hour. Three hours later, its radioactivity, compared with that at the beginning of the first hour, is
A) 1/2.
B) 3/2.
C) 1/4.
D) 1/6.
E) 1/8.
A) 1/2.
B) 3/2.
C) 1/4.
D) 1/6.
E) 1/8.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
37
If 30 g of substance remain from an original sample of 240 g after 600 days, the half-life of the substance is
A) 50 days.
B) 70 days.
C) 100 days.
D) 200 days.
E) 400 days.
A) 50 days.
B) 70 days.
C) 100 days.
D) 200 days.
E) 400 days.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
38
A certain radioisotope has a lifetime of 40 days. The time it will take for 3/4 of the originally present nuclei to disintegrate is
A) 17 days.
B) 30 days.
C) 40 days.
D) 63 days.
E) 80 days.
A) 17 days.
B) 30 days.
C) 40 days.
D) 63 days.
E) 80 days.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
39
If 40 g of substance of half-life 5.0 hours remain from an original sample of 1.28 kg, the time that has passed is
A) 1.0 hour.
B) 20 hours.
C) 25 hours.
D) 80 hours.
E) 160 hours.
A) 1.0 hour.
B) 20 hours.
C) 25 hours.
D) 80 hours.
E) 160 hours.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
40
After 4.5 days, 50 g of substance remain from an original sample having a half-life of 1.5 hours. The original amount of substance was
A) 17 g.
B) 50 g.
C) 150 g.
D) 400 g.
E) 600 g.
A) 17 g.
B) 50 g.
C) 150 g.
D) 400 g.
E) 600 g.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
41
At a particular time, a radioactive source A has a strength of 1.60 *1011 Bq and a half-life of 8.00 days, and a radioactive source B has a strength of 6.40 *1011 Bq. The two sources have the same strength 30.0 days later. The half-life of source B is
A) 35.7 days.
B) 24.7 days.
C) 17.2 days.
D) 7.53 days.
E) 5.22 days.
A) 35.7 days.
B) 24.7 days.
C) 17.2 days.
D) 7.53 days.
E) 5.22 days.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
42
The graph here shows the number of undecayed nuclei as a function of time for a radioactive element. The half-life of this particular radioisotope is 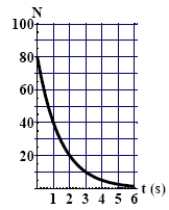
A) 1.0 s.
B) 2.0 s.
C) 4.0 s.
D) 6.0 s.
E) More information is needed to work out the answer.

A) 1.0 s.
B) 2.0 s.
C) 4.0 s.
D) 6.0 s.
E) More information is needed to work out the answer.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
43
The graph here shows the number of undecayed nuclei as a function of time for a radioactive element. The number of undecayed nuclei at t = 16 s is 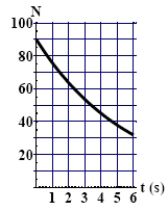
A) 30
B) 23
C) 13
D) 5.6
E) More information is needed to work out the answer.

A) 30
B) 23
C) 13
D) 5.6
E) More information is needed to work out the answer.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
44
The graphs here show the number of undecayed nuclei as a function of time for a radioactive element. The graphs corresponding to elements having the same half-life are 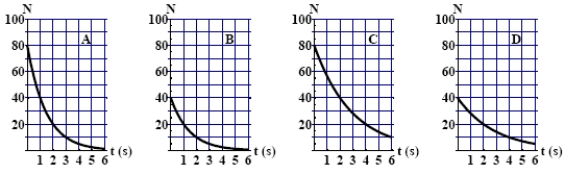
A) A and B.
B) A and C.
C) A and D.
D) B and C.
E) B and D.

A) A and B.
B) A and C.
C) A and D.
D) B and C.
E) B and D.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
45
The graphs here show the number of undecayed nuclei as a function of time for a radioactive element. The graph corresponding to elements having the smaller decay constant is
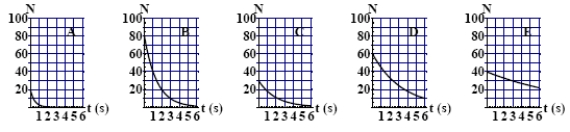
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck
46
The radioactivity due to 14C (half-life 5730 years) measured in a piece of a wooden casket from an ancient burial site was found to produce 10 counts per minute from a given sample, whereas the same amount of carbon from a piece of living wood produced 160 counts per minute. The estimated age of the artifact is
A) 358 years.
B) 5,700 years.
C) 22,900 years.
D) 45,800 years.
E) 91,700 years.
A) 358 years.
B) 5,700 years.
C) 22,900 years.
D) 45,800 years.
E) 91,700 years.

Unlock Deck
Unlock for access to all 46 flashcards in this deck.
Unlock Deck
k this deck


