Deck 19: The Ideal Gas
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/50
Play
Full screen (f)
Deck 19: The Ideal Gas
1
Boyle's Law states that
A) p + V = constant.
B) p - V = constant.
C) P.V = constant.
D) p/V = constant.
A) p + V = constant.
B) p - V = constant.
C) P.V = constant.
D) p/V = constant.
P.V = constant.
2
Charles' Law states that
A) P.T = constant.
B) p/T = constant.
C) VT = constant.
D) V/T = constant.
A) P.T = constant.
B) p/T = constant.
C) VT = constant.
D) V/T = constant.
V/T = constant.
3
According to the ideal gas law, pV = constant for a given temperature. As a result, an increase in volume corresponds to a decrease in pressure. This happens because the molecules
A) move more slowly on the average.
B) collide with each other more frequently.
C) strike the container wall less often.
D) transfer less energy to the walls of the container each time they strike it.
A) move more slowly on the average.
B) collide with each other more frequently.
C) strike the container wall less often.
D) transfer less energy to the walls of the container each time they strike it.
strike the container wall less often.
4
For a given temperature and pressure, equal volumes of different gases have the same
A) number of molecules.
B) molecular weight.
C) density.
D) total mass.
A) number of molecules.
B) molecular weight.
C) density.
D) total mass.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
5
Two gases at the same temperature must have the same
A) pressure.
B) volume.
C) density.
D) number of molecules.
E) kinetic energy per molecule.
A) pressure.
B) volume.
C) density.
D) number of molecules.
E) kinetic energy per molecule.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
6
In the lungs, the respiratory membrane separates tiny (spherical) sacs of air called alveoli (pressure 1.0 105 N/m2, temperature 310 K, and radius 0.11 mm). The air in the lungs contains 14% oxygen. The number of oxygen molecules in one sac is
A) 9.3 1014.
B) 4.7 1014.
C) 1.3 1014.
D) 1.8 1013.
E) 9.0 1012.
A) 9.3 1014.
B) 4.7 1014.
C) 1.3 1014.
D) 1.8 1013.
E) 9.0 1012.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
7
The number of atoms in a 2.0 cm 2.0 cm 2.0 cm cube of copper (molecular mass 29 and density 8920 kg/m3) is
A) 2.3 1022.
B) 1.5 1024.
C) 2.46 1024.
D) 1.5 1027.
E) 2.46 1027.
A) 2.3 1022.
B) 1.5 1024.
C) 2.46 1024.
D) 1.5 1027.
E) 2.46 1027.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
8
A solution contains 40 mg/cm3 of pure protein at 150 Pa and 300 K. The molar mass of the protein is
A) 1 u.
B) 1.7 u.
C) 6.6 u.
D) 1.7 105 u.
E) 6.6 105 u.
A) 1 u.
B) 1.7 u.
C) 6.6 u.
D) 1.7 105 u.
E) 6.6 105 u.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
9
Equal masses of nitrogen and oxygen (molecular masses about 28 and 32 respectively) are placed in two different containers having the same volume. The pressure of the gases in the two containers is the same. If the temperature of the oxygen is T1, the temperature of the nitrogen is
A) larger than T1.
B) T1.
C) smaller than T1.
D) 0o C.
A) larger than T1.
B) T1.
C) smaller than T1.
D) 0o C.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
10
The number of molecules in a helium (He) sample that occupies 50 at STP is
A) 6.7 1018.
B) 3.4 1021.
C) 2.0 1024.
D) 1.3 1024.
E) 1.3 1018.
A) 6.7 1018.
B) 3.4 1021.
C) 2.0 1024.
D) 1.3 1024.
E) 1.3 1018.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
11
The number of water molecules in 50 g of water (expressed in terms of Avogadro's number) is
A) 900 NA.
B) 50 NA.
C) 18 NA.
D) 2.8 NA.
E) 0.36 NA.
A) 900 NA.
B) 50 NA.
C) 18 NA.
D) 2.8 NA.
E) 0.36 NA.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
12
Tylenol is a common painkiller containing acetaminophen (C8H9NO2). One mole of acetaminophen contains Avogadro's number of
A) carbon atoms.
B) hydrogen atoms.
C) nitrogen atoms.
D) oxygen atoms.
E) none of the above.
A) carbon atoms.
B) hydrogen atoms.
C) nitrogen atoms.
D) oxygen atoms.
E) none of the above.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
13
A tank of constant volume is filled with oxygen at temperature T (in Kelvin) to a pressure p. To double the pressure in the tank and halve the temperature, the fraction of the oxygen that should be removed is
A) 0%.
B) 25%.
C) 50%.
D) 75%.
A) 0%.
B) 25%.
C) 50%.
D) 75%.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
14
A storage tank at STP contains 30 kg of oxygen (O2). If an additional 15 kg of oxygen is added to the tank without changing the temperature, the pressure in the storage tank becomes
A) 0.51 105 N/m2.
B) 1.0 105 N/m2.
C) 1.5 105 N/m2.
D) 2.0 105 N/m2.
E) 3.0 105 N/m2.
A) 0.51 105 N/m2.
B) 1.0 105 N/m2.
C) 1.5 105 N/m2.
D) 2.0 105 N/m2.
E) 3.0 105 N/m2.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
15
Tylenol is a common painkiller containing 325 mg acetaminophen (C8H9NO2). Advil is another common painkiller containing 200 mg of ibuprofen (C13H18O2). The ratio of the number of molecules in Tylenol to that in Advil is
A) 0.45.
B) 0.48.
C) 2.08.
D) 2.22.
A) 0.45.
B) 0.48.
C) 2.08.
D) 2.22.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
16
Oxygen is delivered to hospitals in special tanks at 65.0 atm and 288 K. The oxygen is pumped to the patient's room, and it is delivered at 1.00 atm and 297 K. The volume of oxygen produced by 1.00 of tank oxygen when being administered in the patient's room is
A) 7.22
B) 58.5
C) 63.0
D) 67.0
E) 131
A) 7.22
B) 58.5
C) 63.0
D) 67.0
E) 131

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
17
Each of the lungs holds 2.0 of air at the physiological temperature of 37oC. The number of moles of air in the lungs (where the gauge pressure is 21 kPa) is
A) 1.9 10-3.
B) 1.9 10-1.
C) 1.9.
D) 1.9 103.
E) 1.6 106.
A) 1.9 10-3.
B) 1.9 10-1.
C) 1.9.
D) 1.9 103.
E) 1.6 106.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
18
A tire is filled with air at 20oC to a gauge pressure of 200 kPa. Then the tire reaches a temperature of 40oC. The fraction of the original air that must be removed if the original pressure of 200 kPa is to be maintained is
A) 6.4%.
B) 6.8%.
C) 50.0%.
D) 93.2%.
E) 93.6%.
A) 6.4%.
B) 6.8%.
C) 50.0%.
D) 93.2%.
E) 93.6%.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
19
When applying the ideal gas law, we express temperature in units of
A) degrees Celsius.
B) degrees Fahrenheit.
C) Kelvin.
D) any of the above.
E) none of the above.
A) degrees Celsius.
B) degrees Fahrenheit.
C) Kelvin.
D) any of the above.
E) none of the above.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
20
Sometimes it is asserted that at absolute zero "all motion ceases." The intermolecular forces at very low temperatures that support this assertion
A) have a range greater than zero.
B) may produce liquefaction of the gas.
C) may produce solidification of the gas.
D) All of the above are true.
E) None of the above is true.
A) have a range greater than zero.
B) may produce liquefaction of the gas.
C) may produce solidification of the gas.
D) All of the above are true.
E) None of the above is true.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
21
When asked to build a device that measures temperature, you investigate the thermal properties of certain materials. The following property can be used:
A) Electrical resistance of an electrical conductor.
B) The length of a column of liquid.
C) Temperature induced color transition of a material.
D) The volume of a gas.
E) All of the above.
A) Electrical resistance of an electrical conductor.
B) The length of a column of liquid.
C) Temperature induced color transition of a material.
D) The volume of a gas.
E) All of the above.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
22
The quantity that changes when we measure temperature with a constant-volume gas thermometer is
A) the volume of the gas.
B) the pressure of the gas.
C) the number of moles of gas.
D) the number of molecules of gas.
E) none of the above.
A) the volume of the gas.
B) the pressure of the gas.
C) the number of moles of gas.
D) the number of molecules of gas.
E) none of the above.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
23
The curve that correctly represents the relationship between the Fahrenheit and Celsius scales in a temperature (oF) versus temperature (oC) graph is
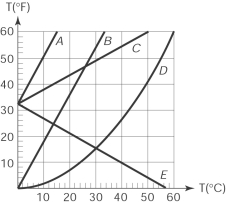
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
24
The curve that correctly represents the relationship between the Fahrenheit and Celsius scales in a temperature (oC) versus temperature (oF) graph is
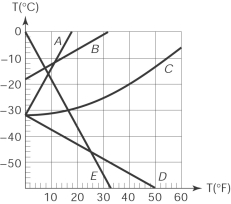
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
25
The temperature changes from 40oF during the night to 70oF during the day. The temperature change on the Celsius scale is
A) 17oC.
B) 30oC.
C) 34oC.
D) 49oC.
E) 54oC.
A) 17oC.
B) 30oC.
C) 34oC.
D) 49oC.
E) 54oC.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
26
The surface water temperature on a lake is T. A thermometer is lowered several meters into the lake. The temperature recorded is
A) larger than T.
B) T.
C) smaller than T.
A) larger than T.
B) T.
C) smaller than T.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
27
After we place ice in a glass of water, the temperature decreases from 15.0oC to 5.0oC. The change in temperature is
A) 5.0 K.
B) 10.0 K.
C) 278 K.
D) 285 K.
E) 288 K.
A) 5.0 K.
B) 10.0 K.
C) 278 K.
D) 285 K.
E) 288 K.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
28
After we place ice in a glass of water, the temperature decreased from 15.0oC to 5.0oC. The change in temperature is
A) 10oF.
B) 14oF.
C) 18oF.
D) 22oF.
E) 42oF.
A) 10oF.
B) 14oF.
C) 18oF.
D) 22oF.
E) 42oF.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
29
The correct ranking of the magnitudes of the average speed, the most probable speed, and the root-mean-square speed for an ideal gas is
A) average < most probable < root-mean-square.
B) root-mean-square < average < most probable.
C) root-mean-square < most probable < average.
D) most probable < root-mean-square < average.
E) most probable < average < root-mean-square.
A) average < most probable < root-mean-square.
B) root-mean-square < average < most probable.
C) root-mean-square < most probable < average.
D) most probable < root-mean-square < average.
E) most probable < average < root-mean-square.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
30
At room temperature, the rms speed of molecules of an ordinary gas is on the order of
A) the speed of light in air.
B) the speed of sound in air.
C) the speed of an Olympic running star.
D) the speed of a person walking.
E) the speed of a tortoise.
A) the speed of light in air.
B) the speed of sound in air.
C) the speed of an Olympic running star.
D) the speed of a person walking.
E) the speed of a tortoise.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
31
For two gases at the same temperature, the rms speed of the molecules with the smaller mass is
A) greater than that of the molecules with the larger mass.
B) equal to that of the molecules with the larger mass.
C) smaller than that of the molecules with the larger mass.
D) unknown; more information is needed to work-out the answer.
A) greater than that of the molecules with the larger mass.
B) equal to that of the molecules with the larger mass.
C) smaller than that of the molecules with the larger mass.
D) unknown; more information is needed to work-out the answer.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
32
When the average speed of the molecules in a given sample of gas is doubled, the temperature is
A) unchanged.
B) increased by a factor of
C) increased by a factor of 2.
D) increased by a factor of 3.
E) increased by a factor of 4.
A) unchanged.
B) increased by a factor of
C) increased by a factor of 2.
D) increased by a factor of 3.
E) increased by a factor of 4.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
33
The temperature (in kelvins) of a gas is doubled. An increase of a factor is observed in
A) the rms speed.
B) the average speed.
C) the most probable speed.
D) All of the above are correct.
E) None of the above is correct.
A) the rms speed.
B) the average speed.
C) the most probable speed.
D) All of the above are correct.
E) None of the above is correct.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
34
An ideal gas is slowly expanding to twice its original volume with no change in temperature. The average speed of the molecules in the sample is
A) increasing by a factor of 2.
B) increasing by a factor of
C) staying unchanged.
D) decreasing by a factor of
E) decreasing by a factor of 2.
A) increasing by a factor of 2.
B) increasing by a factor of
C) staying unchanged.
D) decreasing by a factor of
E) decreasing by a factor of 2.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
35
If the rms speed in a particular gas is doubled, the temperature of the gas is
A) halved.
B) unchanged.
C) doubled.
D) tripled.
E) quadrupled.
A) halved.
B) unchanged.
C) doubled.
D) tripled.
E) quadrupled.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
36
The equivalent of the root-mean-square speed for an ideal gas is
A) the average speed of a molecule in the gas.
B) the speed of a molecule possessing the average kinetic energy in the gas.
C) the speed of a molecule possessing the average momentum in the gas.
D) All of the above are correct.
E) None of the above is correct.
A) the average speed of a molecule in the gas.
B) the speed of a molecule possessing the average kinetic energy in the gas.
C) the speed of a molecule possessing the average momentum in the gas.
D) All of the above are correct.
E) None of the above is correct.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
37
There are 5.00 1026 atoms/m3 in a container of argon gas (atomic mass number 40). The pressure in the container when the atoms have a rms speed of 350 m/s is
A) 0.453 105 N/m2.
B) 1.01 104 N/m2.
C) 1.01 105 N/m2.
D) 1.36 105 N/m2.
E) 1.36 106 N/m2.
A) 0.453 105 N/m2.
B) 1.01 104 N/m2.
C) 1.01 105 N/m2.
D) 1.36 105 N/m2.
E) 1.36 106 N/m2.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
38
The figure here shows the distribution of molecular speeds of a gas for three temperatures T1, T2 and T3. The ranking of the temperatures is
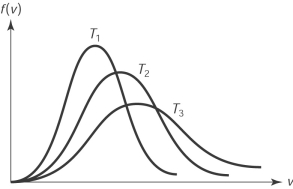
A) T1 < T2 < T3.
B) T1 > T2 > T3.
C) T2 < T1< T3.
D) T2 > T1> T3.
E) T1= T2 = T3.

A) T1 < T2 < T3.
B) T1 > T2 > T3.
C) T2 < T1< T3.
D) T2 > T1> T3.
E) T1= T2 = T3.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
39
The figure here shows the distribution of molecular speeds of a gas for two temperatures T1 and T2. The speed is
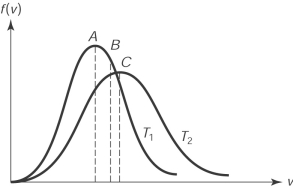
A) the rms speed of the molecules whose temperature is T1.
B) the rms speed of the molecules whose temperature is T2.
C) the average speed of the molecules whose temperature is T1.
D) the average speed of the molecules whose temperature is T2.
E) the most probable speed of the molecules whose temperature is T1.

A) the rms speed of the molecules whose temperature is T1.
B) the rms speed of the molecules whose temperature is T2.
C) the average speed of the molecules whose temperature is T1.
D) the average speed of the molecules whose temperature is T2.
E) the most probable speed of the molecules whose temperature is T1.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
40
The molecules of a five-particle gas have these velocities: 3.0i + 4.0j, - 2.0i + j, - 5.0j, i - 2.0j, and -2.0i + 2.0j. The rms speed of the gas is
A) 0.0 m/s.
B) 1.73 m/s.
C) 3.46 m/s.
D) 4.12 m/s.
E) 8.24 m/s.
A) 0.0 m/s.
B) 1.73 m/s.
C) 3.46 m/s.
D) 4.12 m/s.
E) 8.24 m/s.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
41
The molecules of a five-particle gas have these velocities: 3.0i + 4.0j, - 2.0i + j, - 5.0j, i - 2.0j, and -2.0i + 2.0j. The average speed of the gas is
A) 0.0 m/s.
B) 1.73 m/s.
C) 3.46 m/s.
D) 4.12 m/s.
E) 8.24 m/s.
A) 0.0 m/s.
B) 1.73 m/s.
C) 3.46 m/s.
D) 4.12 m/s.
E) 8.24 m/s.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
42
The molecules of a five-particle gas have these velocities: 3.0i + 4.0j, - 2.0i + j, - 5.0j, i - 2.0j, and -2.0i + 2.0j. The average velocity of the gas is
A) 0.0 m/s.
B) 1.73i m/s.
C) 3.46j m/s.
D) 4.12j m/s.
E) 8.24i m/s.
A) 0.0 m/s.
B) 1.73i m/s.
C) 3.46j m/s.
D) 4.12j m/s.
E) 8.24i m/s.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
43
The mass of a deuterium (diatomic) molecule is roughly the same as that of an ordinary helium (monatomic) atom. If deuterium gas is at the same temperature as helium gas, the rms speeds of the deuterium molecules is
A) larger than the rms speed of the helium atoms.
B) approximately the same as the rms speed of the helium atoms.
C) smaller than the rms speed of the helium atoms.
D) unknown; more information is needed to work out the answer.
A) larger than the rms speed of the helium atoms.
B) approximately the same as the rms speed of the helium atoms.
C) smaller than the rms speed of the helium atoms.
D) unknown; more information is needed to work out the answer.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
44
The mass of a deuterium (diatomic) molecule is roughly the same as that of an ordinary helium (monatomic) atom. If deuterium gas is at the same temperature as helium gas, the average kinetic energy of the deuterium molecules is
A) larger than the average kinetic energy of the helium atoms.
B) approximately the same as the average kinetic energy of the helium atoms.
C) smaller than the average kinetic energy of the helium atoms.
D) unknown; more information is needed to work out the answer.
A) larger than the average kinetic energy of the helium atoms.
B) approximately the same as the average kinetic energy of the helium atoms.
C) smaller than the average kinetic energy of the helium atoms.
D) unknown; more information is needed to work out the answer.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
45
Equal numbers of moles of nitrogen and oxygen (molecular masses about 28 and 32 respectively) are placed in two different containers. The nitrogen molecules are kept at 20oC. If the oxygen and nitrogen molecules have the same rms speed, the temperature of the container with oxygen molecules is
A) 20oC.
B) 23oC.
C) 31oC.
D) 62oC.
E) 93oC.
A) 20oC.
B) 23oC.
C) 31oC.
D) 62oC.
E) 93oC.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
46
The ranking of the thermal energies of n moles of hydrogen (K1), helium (K2), and oxygen (K3) at STP is
A) K1 = K2 = K3.
B) K1 < K2 < K3
C) K1 < K2 < K3.
D) K2 < K1 < K3
E) K2 < K3 < K1.
A) K1 = K2 = K3.
B) K1 < K2 < K3
C) K1 < K2 < K3.
D) K2 < K1 < K3
E) K2 < K3 < K1.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
47
The atmosphere of the Sun consists mostly of hydrogen atoms at 6.0 103 K. The thermal energy per atom is
A) 1.2 10-22 J/atom.
B) 1.2 10-19 J/atom.
C) 1.2 10-16 J/atom.
D) 5.0 104 J/atom.
E) 5.0 107 J/atom.
A) 1.2 10-22 J/atom.
B) 1.2 10-19 J/atom.
C) 1.2 10-16 J/atom.
D) 5.0 104 J/atom.
E) 5.0 107 J/atom.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
48
Interstellar space, far from any stars, is filled with hydrogen atoms (1.0 atom/cm3) at 3.0 K. The pressure in the interstellar space is
A) 2.7 10-23 N/m2.
B) 4.1 10-20 N/m2.
C) 4.1 10-23 N/m2.
D) 6.2 10-20 N/m2.
E) 6.2 10-23 N/m2.
A) 2.7 10-23 N/m2.
B) 4.1 10-20 N/m2.
C) 4.1 10-23 N/m2.
D) 6.2 10-20 N/m2.
E) 6.2 10-23 N/m2.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
49
A cubic box of volume 125 cm3 is filled with air at atmospheric pressure and temperature 20oC. The box is closed and heated to 120oC. The net force on each side of the box is
A) 1.1 N.
B) 1.1 102 N.
C) 3.6 102 N.
D) 1.0 103 N.
E) 1.3 103 N.
A) 1.1 N.
B) 1.1 102 N.
C) 3.6 102 N.
D) 1.0 103 N.
E) 1.3 103 N.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck
50
A 3.0 m 3.0 m 4.0 m room is filled with air (80% N2 and 20% O2 of the total volume) at STP. The thermal energy of the air in the room is
A) 3.6 106 J.
B) 4.4 106 J.
C) 5.5 106 J.
D) 7.2 106 J.
E) 9.0 106 J.
A) 3.6 106 J.
B) 4.4 106 J.
C) 5.5 106 J.
D) 7.2 106 J.
E) 9.0 106 J.

Unlock Deck
Unlock for access to all 50 flashcards in this deck.
Unlock Deck
k this deck


