Deck 3: Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/82
Play
Full screen (f)
Deck 3: Compounds
1
Which of the following is a cation?
A) Ca2+
B) Na
C) Br
D) O2-
A) Ca2+
B) Na
C) Br
D) O2-
Ca2+
2
Which of the following is an anion?
A) F
B) Al3+
C) Mg
D) I-
A) F
B) Al3+
C) Mg
D) I-
I-
3
CO32- is the ___ ion.
A) acetate
B) hydrogencarbonate (bicarbonate)
C) carbonate
D) cyanide
A) acetate
B) hydrogencarbonate (bicarbonate)
C) carbonate
D) cyanide
carbonate
4
The formula of a phosphate ion is ___.
A) PO43-
B) P3-
C) HPO42-
D) H2PO4-
A) PO43-
B) P3-
C) HPO42-
D) H2PO4-

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
5
The name for the Cu2+ ion is
A) copper ion.
B) cobalt (II) ion.
C) copper (I) ion.
D) copper(II) ion.
A) copper ion.
B) cobalt (II) ion.
C) copper (I) ion.
D) copper(II) ion.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
6
NO3- is the ___ ion.
A) nitrous
B) nitrogen trioxide
C) nitrite
D) nitrate
A) nitrous
B) nitrogen trioxide
C) nitrite
D) nitrate

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
7
HPO42- is the ___ ion.
A) hydrogen phosphate
B) monophosphate
C) phosphide
D) phosphite
A) hydrogen phosphate
B) monophosphate
C) phosphide
D) phosphite

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
8
Which symbol is representative of a halide ion?
A) He
B) Ne
C) I-
D) Fe2+
A) He
B) Ne
C) I-
D) Fe2+

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
9
The name for the Ti4+ ion is
A) Tin ion.
B) Tin (IV) ion.
C) Titanium ion.
D) Titanium (IV) ion.
A) Tin ion.
B) Tin (IV) ion.
C) Titanium ion.
D) Titanium (IV) ion.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
10
The formation of ions typically occurs when
A) nonmetal atoms lose electrons and metals gain them.
B) metal and nonmetal atoms are close together in the number of valence electrons.
C) metal and nonmetal atoms have the same number of electrons.
D) nonmetal atoms gain electrons and metal atoms lose them.
A) nonmetal atoms lose electrons and metals gain them.
B) metal and nonmetal atoms are close together in the number of valence electrons.
C) metal and nonmetal atoms have the same number of electrons.
D) nonmetal atoms gain electrons and metal atoms lose them.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
11
Which is the appropriate description of Sr2+?
A) This is an atom and can be used to form chemical compounds.
B) This is an atom with a charge of +2 because there are more neutrons than protons.
C) This is an ion with a charge of +2 because there are two more protons than electrons.
D) This is an ion with a charge of +2 because there are more electrons than protons.
A) This is an atom and can be used to form chemical compounds.
B) This is an atom with a charge of +2 because there are more neutrons than protons.
C) This is an ion with a charge of +2 because there are two more protons than electrons.
D) This is an ion with a charge of +2 because there are more electrons than protons.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
12
Nitrogen can have an anion, N3-. This ion
A) is the result of the atom gaining three electrons.
B) can form three ionic bonds.
C) has eight electrons in the outside orbit.
D) All of these choices are correct.
A) is the result of the atom gaining three electrons.
B) can form three ionic bonds.
C) has eight electrons in the outside orbit.
D) All of these choices are correct.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
13
How many electrons are contained in sulfide ion? 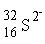
A) 18
B) 14
C) 30
D) 16
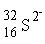
A) 18
B) 14
C) 30
D) 16

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
14
Which ion has the same number of electrons as the noble gas argon?
A) F-
B) Mg2+
C) Br-
D) Ca2+
A) F-
B) Mg2+
C) Br-
D) Ca2+

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
15
Choose the electron dot structure for a magnesium atom.
A)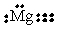
B)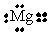
C)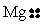
D)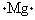
A)
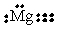
B)
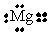
C)
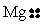
D)
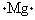

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
16
According to the octet rule, how many valence electrons are present in a chloride ion (Cl-)?
A) 1
B) 2
C) 8
D) 4
A) 1
B) 2
C) 8
D) 4

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
17
How many valence electrons must S atom gain to reach an octet?
A) 1
B) 2
C) 3
D) 0
A) 1
B) 2
C) 3
D) 0

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
18
According to the octet rule, strontium (Sr) will form which of the following monoatomic ions?
A) Sr2-
B) Sr3+
C) Sr2+
D) Sr3-
A) Sr2-
B) Sr3+
C) Sr2+
D) Sr3-

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
19
Generally, the octet rule works fairly well for predicting the behavior of atoms. However, there is an exception:
A) The halogens do not form ions on the basis of the octet rule.
B) The alkali metals only conform to the octet rule when producing neutral atoms.
C) The transition metals do not necessarily conform to the octet rule.
D) The nonmetals do not conform to the octet rule.
A) The halogens do not form ions on the basis of the octet rule.
B) The alkali metals only conform to the octet rule when producing neutral atoms.
C) The transition metals do not necessarily conform to the octet rule.
D) The nonmetals do not conform to the octet rule.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
20
When considering the octet rule, the explanation of the O2- ion is
A) a loss of two electrons to get closer to 8 in the energy level.
B) when an oxygen atom gains two electrons, it has the same electron arrangement as neon, an inert gas.
C) oxygen's nuclear charge of 8 can only hold 8 electrons in the outer shell.
D) oxygen is an exception to the octet rule.
A) a loss of two electrons to get closer to 8 in the energy level.
B) when an oxygen atom gains two electrons, it has the same electron arrangement as neon, an inert gas.
C) oxygen's nuclear charge of 8 can only hold 8 electrons in the outer shell.
D) oxygen is an exception to the octet rule.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
21
A compound is
A) two or more elements well mixed.
B) two or more elements combined chemically.
C) two or more elements carrying a positive charge.
D) a physical blend of two or more elements.
A) two or more elements well mixed.
B) two or more elements combined chemically.
C) two or more elements carrying a positive charge.
D) a physical blend of two or more elements.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
22
Which pair of elements cannot combine to form a chemical compound?
A) Na and Ne
B) Li and O
C) Fe and S
D) Ca and I
A) Na and Ne
B) Li and O
C) Fe and S
D) Ca and I

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following atom pairs would most likely be connected by an ionic bond?
A) K and Br
B) O and Br
C) S and Br
D) F and Br
A) K and Br
B) O and Br
C) S and Br
D) F and Br

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
24
Calcium chloride has the formula CaCl2. As an ionic compound it is
A) composed of one cation and 2 anions.
B) held together by means of covalent bonds.
C) composed of two cations and one anion.
D) more likely a molecular compound.
A) composed of one cation and 2 anions.
B) held together by means of covalent bonds.
C) composed of two cations and one anion.
D) more likely a molecular compound.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
25
Chose the formula of cobalt(II) nitrate.
A) CoN
B) Co2 NO3
C) Co(NO3)2
D) Co(II)(NO3)2
A) CoN
B) Co2 NO3
C) Co(NO3)2
D) Co(II)(NO3)2

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
26
What is the formula of beryllium sulfide?
A) BeS
B) Be2S
C) Be2S3
D) Be3S2
A) BeS
B) Be2S
C) Be2S3
D) Be3S2

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
27
What is the proper name of the compound FePO4?
A) iron phosphide
B) iron(III) phosphide
C) iron(III) phosphate
D) iron phosphorus tetraoxide
A) iron phosphide
B) iron(III) phosphide
C) iron(III) phosphate
D) iron phosphorus tetraoxide

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
28
What is the formula of the ionic compound that forms between lithium ions and dichromate ions?
A) LiCr2O7
B) Li3 Cr2O7
C) Li2 Cr2O7
D) Li4Cr2O7
A) LiCr2O7
B) Li3 Cr2O7
C) Li2 Cr2O7
D) Li4Cr2O7

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
29
The name of the compound Na2CO3 is
A) sodium carbonate.
B) disodium carbonate.
C) sodium bicarbonate.
D) disodium bicarbonate.
A) sodium carbonate.
B) disodium carbonate.
C) sodium bicarbonate.
D) disodium bicarbonate.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is an ionic compound?
A) CS2
B) CO2
C) Mg(OH)2
D) CH4
A) CS2
B) CO2
C) Mg(OH)2
D) CH4

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
31
What is the formula of the ionic compound that forms between calcium ions and phosphate ions?
A) CaPO4
B) Ca2PO4
C) Ca2(PO4)3
D) Ca3(PO4)2
A) CaPO4
B) Ca2PO4
C) Ca2(PO4)3
D) Ca3(PO4)2

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
32
The difference between an ionic bond and a covalent bond is that
A) ionic bonds commonly occur between two metals and covalent bonds occur between metals and nonmetals.
B) ionic bonds are between atoms that can share electrons; covalent bonds are between atoms that will donate/accept electrons.
C) covalent bonds come about because of a sharing of electrons; ionic bonds do not.
D) ionic bonds are between smaller atoms; covalent bonds are between large atoms.
A) ionic bonds commonly occur between two metals and covalent bonds occur between metals and nonmetals.
B) ionic bonds are between atoms that can share electrons; covalent bonds are between atoms that will donate/accept electrons.
C) covalent bonds come about because of a sharing of electrons; ionic bonds do not.
D) ionic bonds are between smaller atoms; covalent bonds are between large atoms.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
33
Predict the number of covalent bonds by S atom.
A) 1
B) 2
C) 3
D) 4
A) 1
B) 2
C) 3
D) 4

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is a binary compound?
A) NaOH
B) FeCl3
C) Ca 3(PO4)2
D) None of these choices is a binary compound.
A) NaOH
B) FeCl3
C) Ca 3(PO4)2
D) None of these choices is a binary compound.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
35
The name of the compound N2O4 is
A) nitrogen oxide.
B) nitrogen tetroxide.
C) dinitrogen tetroxide.
D) nitrogen dioxide.
A) nitrogen oxide.
B) nitrogen tetroxide.
C) dinitrogen tetroxide.
D) nitrogen dioxide.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
36
What is the correct name of the compound Cl2O3?
A) dichlorine trioxide
B) Chlorine oxide
C) Chlorine trioxide
D) Chloride oxide
A) dichlorine trioxide
B) Chlorine oxide
C) Chlorine trioxide
D) Chloride oxide

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
37
What is the molar mass of ammonium oxide?
A) 30.00 g/mol
B) 34.00 g/mol
C) 44.00 g/mol
D) 52.00 g/mol
A) 30.00 g/mol
B) 34.00 g/mol
C) 44.00 g/mol
D) 52.00 g/mol

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
38
What is the molar mass of isopentyl acetate, C7H14O2, a compound responsible for the odor of bananas?
A) 130.19 g/mole
B) 98.32 g/mole
C) 146.58 g/mole
D) 119.35 g/mole
E) 13.19 g/mole
A) 130.19 g/mole
B) 98.32 g/mole
C) 146.58 g/mole
D) 119.35 g/mole
E) 13.19 g/mole

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
39
What is the molar mass of aspirin (C9H8O4), a drug often used to relieve minor aches and pains?
A) 165.43 g/mol
B) 106.21 g/mol
C) 88.67 g/mol
D) 180.16 g/mol
A) 165.43 g/mol
B) 106.21 g/mol
C) 88.67 g/mol
D) 180.16 g/mol

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
40
A compound commonly called trisodium phosphate, Na3PO4, is used to clean grease and other organic materials from concrete surfaces. Calculate the formula weight of this cleaner.
A) 70.20 amu
B) 116.70 amu
C) 118.30 amu
D) 164.00 amu
A) 70.20 amu
B) 116.70 amu
C) 118.30 amu
D) 164.00 amu

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
41
Aluminum sulfate, Al2(SO4)3, can be used in water purification. What is the formula weight of this salt?
A) 75.10 amu
B) 342.30 amu
C) 315.30 amu
D) 150.10 amu
A) 75.10 amu
B) 342.30 amu
C) 315.30 amu
D) 150.10 amu

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
42
What is the mass in grams of 3 moles of calcium carbonate, CaCO3, a common antacid?
A) 300 grams
B) 150 grams
C) 100 grams
D) 68 grams
A) 300 grams
B) 150 grams
C) 100 grams
D) 68 grams

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
43
Calculate the mass of 10.00 moles of nickel(II) cyanide, Ni(CN)2.
A) 847.0 grams
B) 987.0 grams
C) 1107 grams
D) 1694 grams
A) 847.0 grams
B) 987.0 grams
C) 1107 grams
D) 1694 grams

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
44
Glycerol, often called glycerine, was used as a skin moisturizer before more commercial preparations were made available. Calculate the weight of 7.85 moles glycerin. 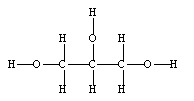
A) 92.3 grams
B) 228 grams
C) 667 grams
D) 722 grams

A) 92.3 grams
B) 228 grams
C) 667 grams
D) 722 grams

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
45
Ethanol, C2H5OH, is found in alcoholic beverages. If a 125-g sample is pure ethanol, how many moles of ethanol are present?
A) 0.419 moles
B) 2.72 moles
C) 5750 moles
D) 46.3 moles
A) 0.419 moles
B) 2.72 moles
C) 5750 moles
D) 46.3 moles

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
46
A 1.0 x 102- gram sample is found to be pure alanine, an amino acid found in proteins. How many moles of alanine are in the sample? 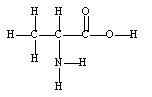
A) 0.20 moles
B) 0.90 moles
C) 1.1 moles
D) 890 moles

A) 0.20 moles
B) 0.90 moles
C) 1.1 moles
D) 890 moles

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
47
What is the mass of 0.00142 mole of vitamin C (C6H8O6)?
A) 0.250 g
B) 0.500 g
C) 0.125 g
D) 2.50 g
A) 0.250 g
B) 0.500 g
C) 0.125 g
D) 2.50 g

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
48
How many molecules of aspirin (C9H8O4) are present in 41.40 g of aspirin?
A) 0.2300 aspirin molecules
B) 2.700 x 1026 aspirin molecules
C) 9.600 x 1023 aspirin molecules
D) 1.383 x 1023 aspirin molecules
A) 0.2300 aspirin molecules
B) 2.700 x 1026 aspirin molecules
C) 9.600 x 1023 aspirin molecules
D) 1.383 x 1023 aspirin molecules

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
49
Ag+ is an ion with 47 protons and 108 electrons.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
50
A Roman numeral is used in the name of transition metal ions. That numeral indicates the charge of the ion.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
51
Helium forms an ion with a 2+ charge.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
52
SO32- is called the sulfur trioxide ion.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
53
Elements in family 8A tend to form anions.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
54
Sodium carbonate is an ionic compound whose formula is NaCO3.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
55
The formula of the ionic compound that forms between aluminum ions and Sulfide ions is AlS2.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
56
Potassium and magnesium cannot form a binary compound together.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
57
The formula weight of magnesium fluoride is 62.30 amu.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
58
The magnesium ___ is represented by Mg2+.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
59
Roman numerals are used to denote the charge of ___ metal cations if they form more than one ionic charge.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
60
The bicarbonate ion is different than the carbonate ion because it contains one ___ not found in the carbonate ion.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
61
Ionic compounds tend to exist in the ___ state at room temperature.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
62
___ is the formula for aluminum fluoride.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
63
The formula of manganese(II) oxide is ___.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
64
The valence electrons not involved in bonds are called ___.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
65
The oxygen gas found in the atmosphere contains ___ atom(s) per molecule.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
66
The risk of heart attack and stroke increase when a person is given blood transfusion because ___ is lacking in stored blood.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
67
Complete the following table:
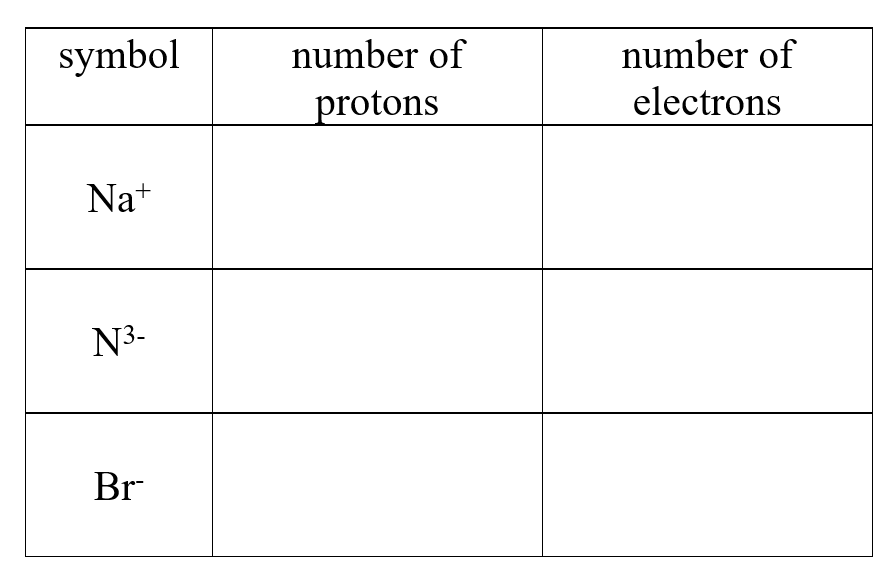


Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
68
Sketch electron dot structures for each of the following atoms:
a) Cl
b) Al
c) Ca
d) N
a) Cl
b) Al
c) Ca
d) N

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
69
Give the name for each of the following:
a) SiF4
b) CuBr2
c) MgCO3
d) NH4NO3
a) SiF4
b) CuBr2
c) MgCO3
d) NH4NO3

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
70
Draw the line bond structure of sulfuric acid.
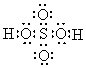


Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
71
Draw a structure expected from the combination of nitrogen and three chlorine atoms?

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
72
Name the following compounds:
a) MgCl2
b) AgNO3
c) Fe(N03)3
d) SCl2
a) MgCl2
b) AgNO3
c) Fe(N03)3
d) SCl2

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
73
Give the formula for the following compounds:
a) diphosphorus triiodide
b) silver oxide
c) cobalt (II) phosphate
d) carbon disulfide
a) diphosphorus triiodide
b) silver oxide
c) cobalt (II) phosphate
d) carbon disulfide

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
74
Give the formula for the following compounds:
a) strontium bromide
b) disulfur trioxide
c) copper(II) nitrite
d) lithium oxide
a) strontium bromide
b) disulfur trioxide
c) copper(II) nitrite
d) lithium oxide

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
75
Give the name for each of the following:
a) N2Br4
b) NH4Cl
c) Fe(NO3)3
d) NaF
a) N2Br4
b) NH4Cl
c) Fe(NO3)3
d) NaF

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
76
Determine the molar mass for each of the following:
a) Ca(NO3)2
b) AgNO3
c) FeBr2
d) SCl2
a) Ca(NO3)2
b) AgNO3
c) FeBr2
d) SCl2

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
77
Calculate the number of moles in 8.61 grams of calcium nitrate.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
78
What is the mass in grams of 1.82 x 10-1 moles of phosphorus trichloride?

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
79
What is the mass of 3.15 moles of calcium sulfate, CaSO4?

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
80
How many moles are in 25.00 g of water?

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck


