Deck 23: Organic Compounds, Polymers, and Biochemicals
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/140
Play
Full screen (f)
Deck 23: Organic Compounds, Polymers, and Biochemicals
1
How many isomers are there of butane, C4H10?
A)1 (no isomers)
B)2
C)3
D)4
E)5
A)1 (no isomers)
B)2
C)3
D)4
E)5
2
2
How many isomers are there of propane, C3H8?
A)1 (no isomers)
B)2
C)3
D)4
E)5
A)1 (no isomers)
B)2
C)3
D)4
E)5
1 (no isomers)
3
How many isomers are there of pentane, C5H12?
A)1 (no isomers)
B)2
C)3
D)4
E)5
A)1 (no isomers)
B)2
C)3
D)4
E)5
3
4
CH3-CH2-O-H and CH3-O-CH3 are a pair of compounds that are
A)allotropes.
B)allomers.
C)isochrones.
D)isomers.
E)allotones.
A)allotropes.
B)allomers.
C)isochrones.
D)isomers.
E)allotones.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
5
CH3-CH2-NH2 and CH3-NH-CH3 are a pair of compounds that are
A)allotropes.
B)allomers.
C)isochrones.
D)isomers.
E)allotones.
A)allotropes.
B)allomers.
C)isochrones.
D)isomers.
E)allotones.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
6
The functional group, -O-H, is found in which one of these types of organic compounds?
A)alkanes
B)alkenes
C)amines
D)alcohols
E)ethers
A)alkanes
B)alkenes
C)amines
D)alcohols
E)ethers

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
7
The functional group, -NH2, is found in which one of these types of organic compounds?
A)alkanes
B)alkenes
C)amines
D)alcohols
E)ethers
A)alkanes
B)alkenes
C)amines
D)alcohols
E)ethers

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
8
The functional group, R-O-R, is found in which one of these types of organic compounds?
A)alkanes
B)alkenes
C)amines
D)alcohols
E)ethers
A)alkanes
B)alkenes
C)amines
D)alcohols
E)ethers

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
9
Draw a structural formula for cyclohexane, a cyclic saturated hydrocarbon (C6H12). How many ?-bonds are in cyclohexane?
A)12
B)16
C)17
D)18
E)20
A)12
B)16
C)17
D)18
E)20

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
10
Draw a structural formula for cyclohexane, a cyclic saturated hydrocarbon (C6H12). How many ?-bonds are in a cyclohexane molecule?
A)0
B)2
C)3
D)4
E)6
A)0
B)2
C)3
D)4
E)6

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
11
How many isomers are there of propene, 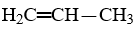 ?
?
A)1 (no isomers)
B)2
C)3
D)4
E)5
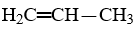 ?
?A)1 (no isomers)
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
12
A saturated 8-carbon straight chain hydrocarbon would have how many hydrogens?
A)8
B)10
C)16
D)18
E)24
A)8
B)10
C)16
D)18
E)24

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
13
Butene, C4H8, is a hydrocarbon with one double bond. How many isomers are there of butane?
A)1 (no isomers)
B)2
C)3
D)4
E)5
A)1 (no isomers)
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
14
Which compound is butane?
A)CH3CH3
B)CH3CH2CH3
C)CH3CH2CH2CH3
D)CH3CH2CH2CH2CH3
E)CH3CH2CH2CH2CH2CH3
A)CH3CH3
B)CH3CH2CH3
C)CH3CH2CH2CH3
D)CH3CH2CH2CH2CH3
E)CH3CH2CH2CH2CH2CH3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
15
Which compound is pentane?
A)CH3CH3
B)CH3CH2CH3
C)CH3CH2CH2CH3
D)CH3CH2CH2CH2CH3
E)CH3CH2CH2CH2CH2CH3
A)CH3CH3
B)CH3CH2CH3
C)CH3CH2CH2CH3
D)CH3CH2CH2CH2CH3
E)CH3CH2CH2CH2CH2CH3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
16
Which compound is a butene?
A)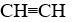
B)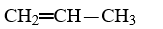
C)CH3-CH2-CH2-CH3
D)CH3-CH2-CH3
E)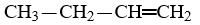
A)
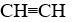
B)
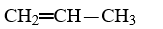
C)CH3-CH2-CH2-CH3
D)CH3-CH2-CH3
E)
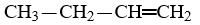

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
17
Which compound is pentane?
A)CH3-CH2-CH2-CH3
B)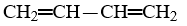
C)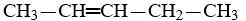
D)CH3-CH2-CH2-CH2-CH3
E)CH3-CH2-CH2-CH2-CH2-CH3
A)CH3-CH2-CH2-CH3
B)
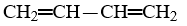
C)
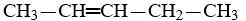
D)CH3-CH2-CH2-CH2-CH3
E)CH3-CH2-CH2-CH2-CH2-CH3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
18
Which one of the groups below goes by the name propyl?
A)CH3-CH2-
B)CH2-CH-
C)CH3-CH2-CH2-
D)CH2-CH-CH2-
E)CH3-CH-CH-
A)CH3-CH2-
B)CH2-CH-
C)CH3-CH2-CH2-
D)CH2-CH-CH2-
E)CH3-CH-CH-

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
19
The alkyl group, -CH3, where the open bond shows where it attaches to a larger carbon chain, is named
A)methide
B)methonium
C)methal
D)methyl
E)trihydrogencarbide
A)methide
B)methonium
C)methal
D)methyl
E)trihydrogencarbide

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
20
The alkyl group shown below, where the open bond shows where it attaches to a larger carbon chain, is named 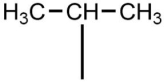
A)propyl
B)propide
C)propanium
D)isopropyl
E)propanyl
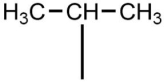
A)propyl
B)propide
C)propanium
D)isopropyl
E)propanyl

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
21
Which compound is not an unsaturated compound?
A)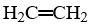
B)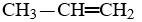
C)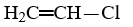
D)CH3-CH2-O-H
E)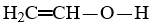
A)
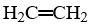
B)
C)
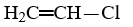
D)CH3-CH2-O-H
E)
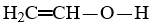

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
22
Which compound is not an unsaturated compound?
A)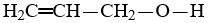
B)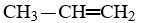
C)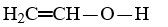
D)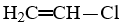
E)CH3-CH2-NH2
A)
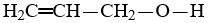
B)
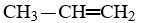
C)
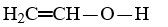
D)
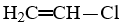
E)CH3-CH2-NH2

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is true about the benzene molecule?
A)It is a straight chain hydrocarbon.
B)It contains a heterocyclic oxygen.
C)It is a saturated hydrocarbon.
D)The double bonds in its ring have resonance and their electrons are delocalized.
E)Attachments to the ring can exhibit cis/trans isomerism.
A)It is a straight chain hydrocarbon.
B)It contains a heterocyclic oxygen.
C)It is a saturated hydrocarbon.
D)The double bonds in its ring have resonance and their electrons are delocalized.
E)Attachments to the ring can exhibit cis/trans isomerism.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
24
In the IUPAC system of nomenclature, the alkane hydrocarbon whose skeleton is shown below, is regarded as a derivative of 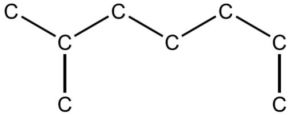
A)butane.
B)hexane.
C)heptane.
D)octane.
E)pentane.

A)butane.
B)hexane.
C)heptane.
D)octane.
E)pentane.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
25
In the IUPAC system of nomenclature, the alkane hydrocarbon whose skeleton is shown below, is regarded as a derivative of 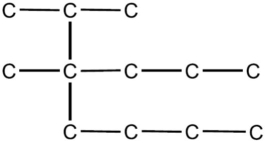
A)dodecane.
B)decane.
C)heptane.
D)pentane.
E)octane.

A)dodecane.
B)decane.
C)heptane.
D)pentane.
E)octane.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
26
The IUPAC name (don't worry about geometric isomers)for the compound, 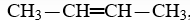 , is
, is
A)butene-2.
B)butene-3.
C)2-butene.
D)2-butyne.
E)3-butene.
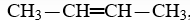 , is
, isA)butene-2.
B)butene-3.
C)2-butene.
D)2-butyne.
E)3-butene.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
27
The IUPAC name for the compound, 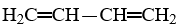 , is
, is
A)butene-2.
B)1.3-dibutene.
C)1,3-butadiene.
D)butane-1,3.
E)2-butynel.
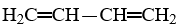 , is
, isA)butene-2.
B)1.3-dibutene.
C)1,3-butadiene.
D)butane-1,3.
E)2-butynel.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
28
If two of the hydrogen atoms in butane are replaced by two chlorine atoms, how many different dichlorobutane isomers can there be?
A)1
B)2
C)3
D)4
E)6
A)1
B)2
C)3
D)4
E)6

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
29
If two of the hydrogen atoms on a single carbon in pentane are replaced by two chlorine atoms, how many different dichloropentane isomers can there be?
A)2
B)3
C)4
D)5
E)6
A)2
B)3
C)4
D)5
E)6

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
30
Draw a structural formula for benzene. How many ?bonds are in the molecule?
A)6
B)12
C)14
D)18
E)20
A)6
B)12
C)14
D)18
E)20

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
31
Draw a structural formula for benzene. How many single and double bonds are in the benzene molecule?
A)3 single, 0 double
B)6 single, 2 double
C)3 single, 2 double
D)9 single, 23 double
E)12 single, 3 double
A)3 single, 0 double
B)6 single, 2 double
C)3 single, 2 double
D)9 single, 23 double
E)12 single, 3 double

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
32
If two of the hydrogen atoms in ethylene, 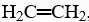 , are replaced by two chlorine atoms to form dichloroethylene, how many different dichloroethylene isomers can there be?
, are replaced by two chlorine atoms to form dichloroethylene, how many different dichloroethylene isomers can there be?
A)1
B)2
C)3
D)4
E)6
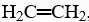 , are replaced by two chlorine atoms to form dichloroethylene, how many different dichloroethylene isomers can there be?
, are replaced by two chlorine atoms to form dichloroethylene, how many different dichloroethylene isomers can there be?A)1
B)2
C)3
D)4
E)6

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
33
If two of the hydrogen atoms in ethylene, 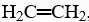 are replaced by one chlorine atom and one fluorine atom to form chlorofluoroethene, C2H2ClF, how many different chlorofluoro-ethene isomers can there be?
are replaced by one chlorine atom and one fluorine atom to form chlorofluoroethene, C2H2ClF, how many different chlorofluoro-ethene isomers can there be?
A)1
B)2
C)3
D)4
E)6
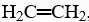 are replaced by one chlorine atom and one fluorine atom to form chlorofluoroethene, C2H2ClF, how many different chlorofluoro-ethene isomers can there be?
are replaced by one chlorine atom and one fluorine atom to form chlorofluoroethene, C2H2ClF, how many different chlorofluoro-ethene isomers can there be?A)1
B)2
C)3
D)4
E)6

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
34
Various hydrocarbons can be separated from a crude oil mixture by
A)filtration.
B)chromatography.
C)precipitation.
D)fractional distillation.
E)centrifugation.
A)filtration.
B)chromatography.
C)precipitation.
D)fractional distillation.
E)centrifugation.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
35
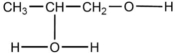
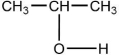
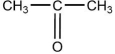
When 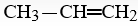 is reacted with water in the presence of a catalytic amount of acid, a new compound is formed. What might be the product of this reaction?
is reacted with water in the presence of a catalytic amount of acid, a new compound is formed. What might be the product of this reaction?
A)CH3-CH-CH2
B)
C)
D)
E)
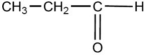
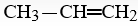 is reacted with water in the presence of a catalytic amount of acid, a new compound is formed. What might be the product of this reaction?
is reacted with water in the presence of a catalytic amount of acid, a new compound is formed. What might be the product of this reaction?A)CH3-CH-CH2
B)

C)

D)

E)
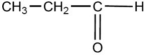

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
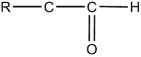
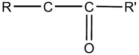
36
The general structural formula for an ether is
A)
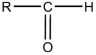
B)
C)
D)
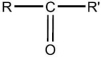
E)R-O-R'
A)

B)

C)

D)

E)R-O-R'

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
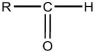
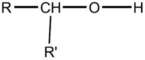
37
Which formula is an alcohol?
A)
B)
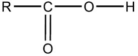
C)
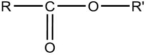
D)
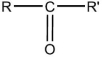
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
38
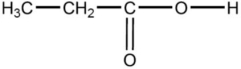
Which is a product of the oxidation of CH3-CH2-CH2-O-H?
A)
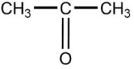
B)
C)CH3-CH2-CH3
D)
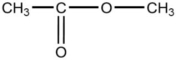
E)CH3-CH2-O-CH3
A)

B)

C)CH3-CH2-CH3
D)

E)CH3-CH2-O-CH3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
39
The functional group 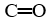 is found in
is found in
A)alkenes.
B)ketones.
C)amines.
D)alcohols.
E)ethers.
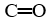 is found in
is found inA)alkenes.
B)ketones.
C)amines.
D)alcohols.
E)ethers.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
40
The functional group 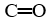 is found in all the species below except
is found in all the species below except
A)aldehydes.
B)ketones.
C)amides.
D)ethers.
E)esters.
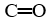 is found in all the species below except
is found in all the species below exceptA)aldehydes.
B)ketones.
C)amides.
D)ethers.
E)esters.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
41
The functional group 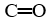 is found in all the species below except
is found in all the species below except
A)aldehydes.
B)carboxylic acids.
C)amides.
D)amines.
E)esters.
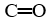 is found in all the species below except
is found in all the species below exceptA)aldehydes.
B)carboxylic acids.
C)amides.
D)amines.
E)esters.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
42
The compound below is classified as which type of compound? 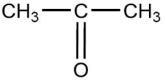
A)aldehyde
B)ketone
C)acid
D)ester
E)amine

A)aldehyde
B)ketone
C)acid
D)ester
E)amine

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
43
The compound below is classified as which type of compound? 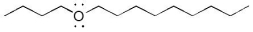
A)amine
B)amide
C)aromatic
D)ester
E)ether

A)amine
B)amide
C)aromatic
D)ester
E)ether

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
44
The compound below is classified as which type of compound? 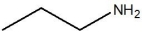
A)amine
B)amide
C)aromatic
D)ester
E)ether

A)amine
B)amide
C)aromatic
D)ester
E)ether

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
45
The compound below is classified as which type of compound? 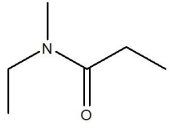
A)amine
B)amide
C)aromatic
D)ester
E)ether

A)amine
B)amide
C)aromatic
D)ester
E)ether

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
46
The compound below is classified as which type of compound? 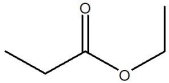
A)amine
B)amide
C)aromatic
D)ester
E)ether

A)amine
B)amide
C)aromatic
D)ester
E)ether

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
47
The compound below has which functional groups? 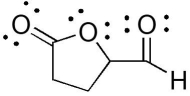
A)ketone and ester
B)ketone, alcohol, and carboxylic acid
C)ether, ketone, and aldehyde
D)ether and aldehyde
E)ketone and aldehyde

A)ketone and ester
B)ketone, alcohol, and carboxylic acid
C)ether, ketone, and aldehyde
D)ether and aldehyde
E)ketone and aldehyde

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
48
The compound below is classified as which type of compound? 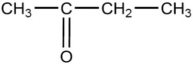
A)aldehyde
B)ketone
C)acid
D)ester
E)amine

A)aldehyde
B)ketone
C)acid
D)ester
E)amine

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
49
The compound below is classified as which type of compound? 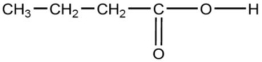
A)ketone
B)aldehyde
C)ester
D)carboxylic acid
E)amide
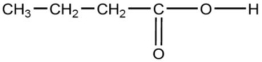
A)ketone
B)aldehyde
C)ester
D)carboxylic acid
E)amide

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
50
The compound below is named 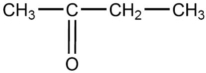
A)butanamine.
B)butanamide.
C)butanketone.
D)2-butanone.
E)2-butanal.

A)butanamine.
B)butanamide.
C)butanketone.
D)2-butanone.
E)2-butanal.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
51
The compound below has which functional groups? 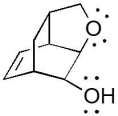
A)aromatic, alcohol, and ether
B)aromatic, alcohol, and ester
C)ether, alkene, and alcohol
D)ester, alkene and alcohol
E)ether and ester

A)aromatic, alcohol, and ether
B)aromatic, alcohol, and ester
C)ether, alkene, and alcohol
D)ester, alkene and alcohol
E)ether and ester

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
52
The compound below has which functional groups? 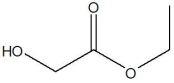
A)alcohol and ester
B)amide, ether, and aldehyde
C)ether and amine
D)ester and amine
E)amine, alcohol, and ether

A)alcohol and ester
B)amide, ether, and aldehyde
C)ether and amine
D)ester and amine
E)amine, alcohol, and ether

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
53
The compound below is named 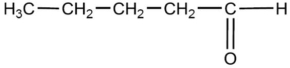
A)pentaketone.
B)pentaldehyde.
C)pentanal.
D)pentanone.
E)pentanoic acid.
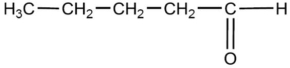
A)pentaketone.
B)pentaldehyde.
C)pentanal.
D)pentanone.
E)pentanoic acid.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
54
The ester that is prepared by heating 1-pentanol with acetic acid in the presence of an acidic catalyst is named
A)acetic pentanoate.
B)pentanoic acetate.
C)1-pentyl acetate.
D)acetyl 1-pentanoate.
E)acetyl pentanol.
A)acetic pentanoate.
B)pentanoic acetate.
C)1-pentyl acetate.
D)acetyl 1-pentanoate.
E)acetyl pentanol.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
55
Ethyl propanoate is a(n)
A)carboxyl alcohol.
B)aldehyde.
C)alcohol.
D)ester.
E)amide.
A)carboxyl alcohol.
B)aldehyde.
C)alcohol.
D)ester.
E)amide.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
56
The compound below has which functional groups? 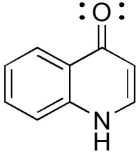
A)ester, amide, carboxylic acid
B)aromatic, ketone, amine
C)aromatic, ketone, amide
D)aromatic, aldehyde, amide
E)aromatic, aldehyde, amine

A)ester, amide, carboxylic acid
B)aromatic, ketone, amine
C)aromatic, ketone, amide
D)aromatic, aldehyde, amide
E)aromatic, aldehyde, amine

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
57
Which is a product of the oxidation of the following: 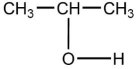
A)
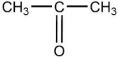
B)
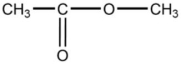
C)CH3-CH2-CH3
D)
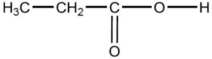
E)CH3-CH2-O-CH3

A)

B)

C)CH3-CH2-CH3
D)

E)CH3-CH2-O-CH3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
58
The reaction of 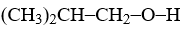 with concentrated HBr using controlled heating yields
with concentrated HBr using controlled heating yields
A)CH3-CH2-CH2-Br
B)(CH3)2CH-CH2-Br
C)(CH3)2CH-CH2-O-Br
D)(CH3)2CH-CH4+ Br-
E)(CH3)2CH-CH3
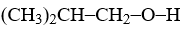 with concentrated HBr using controlled heating yields
with concentrated HBr using controlled heating yieldsA)CH3-CH2-CH2-Br
B)(CH3)2CH-CH2-Br
C)(CH3)2CH-CH2-O-Br
D)(CH3)2CH-CH4+ Br-
E)(CH3)2CH-CH3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
59
Based on the properties of the attached functional group, which compound would you expect to interact most strongly with water, thus making it the substance most soluble in water?Hint: Consider hydrogen bonds.
A)CH3-CH2-Cl
B)CH3-CH2-F
C)CH3-CH2-I
D)CH3-CH2-S-H
E)CH3-CH2-O-H
A)CH3-CH2-Cl
B)CH3-CH2-F
C)CH3-CH2-I
D)CH3-CH2-S-H
E)CH3-CH2-O-H

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
60
Cyclohexyl propanoate is a(n)
A)aldehyde.
B)ketone.
C)carboxylic acid.
D)ester.
E)amide.
A)aldehyde.
B)ketone.
C)carboxylic acid.
D)ester.
E)amide.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
61
Based on the properties of the attached functional group, which compound below would you expect to interact most strongly with water, thus making it the most soluble compound? Hint: Consider hydrogen bonds.
A)CH3-CH2-H
B)CH3-CH2-NH2
C)CH3-O-CH3
D)CH3-CH2-I
E)CH3-CH2-S-H
A)CH3-CH2-H
B)CH3-CH2-NH2
C)CH3-O-CH3
D)CH3-CH2-I
E)CH3-CH2-S-H

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
62
Which compound is least soluble in water?
A)
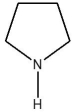
B)
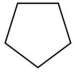
C)
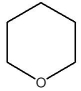
D)
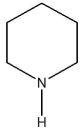
E)
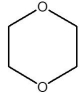
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
63
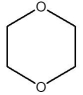
Which compound is least soluble in water?
A)
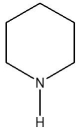
B)
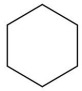
C)
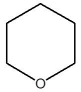
D)
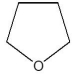
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
64
Which compound is a Brønsted base?
A)
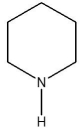
B)
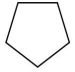
C)
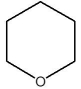
D)
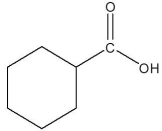
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
65
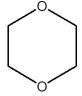
Which one of the species below is soluble in dilute HCl(aq)?
A)
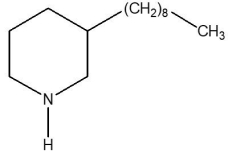
B)
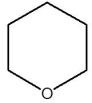
C)
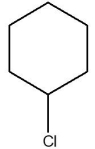
D)
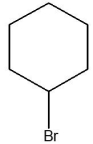
E)
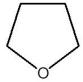
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
66
Which compound is insoluble in water?
A)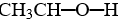
B)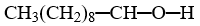
C)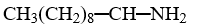
D)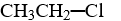
E)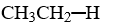
A)
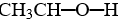
B)
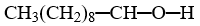
C)
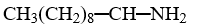
D)
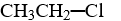
E)
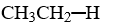

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
67
A functional group containing nitrogen is found in
A)alkenes.
B)alcohols.
C)amines.
D)carboxylic acids.
E)ethers.
A)alkenes.
B)alcohols.
C)amines.
D)carboxylic acids.
E)ethers.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
68
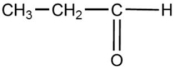
One of the compounds listed is an organic base that functions as a proton acceptor. Identify this organic base.
A)CH3-CH2-CH2-O-H
B)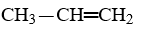
C)
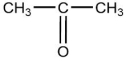
D)
E)CH3-CH2-NH2
A)CH3-CH2-CH2-O-H
B)
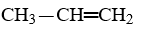
C)

D)

E)CH3-CH2-NH2

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
69
The compound, trimethylamine is a(n)________ and has the formula ________.
A)base, (CH3NH2)3
B)base, (CH3)3NH2
C)acid, (CH3)3NH2
D)base, (CH3)3N
E)acid, (CH3)3(NH2)2
A)base, (CH3NH2)3
B)base, (CH3)3NH2
C)acid, (CH3)3NH2
D)base, (CH3)3N
E)acid, (CH3)3(NH2)2

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
70
Dodecylamine, CH3(CH2)10CH2NH2, is insoluble in water. Yet, it can be converted to a water soluble form. Which of the species below represents a water soluble form of this compound?
A)CH3(CH2)10CH2NH2-O-H
B)CH3(CH2)10CH2NH2-Cl
C)CH3(CH2)10CH2NH-CH3
D)CH3(CH2)10CH2NH3+ Cl-
E)CH3(CH2)10CH2NH2-O-CH3
A)CH3(CH2)10CH2NH2-O-H
B)CH3(CH2)10CH2NH2-Cl
C)CH3(CH2)10CH2NH-CH3
D)CH3(CH2)10CH2NH3+ Cl-
E)CH3(CH2)10CH2NH2-O-CH3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
71
Octylamine, CH3(CH2)7NH2, is insoluble in water. Yet, it can be converted to a water soluble form. Which of the species below represents a water-soluble form of this compound?
A)CH3(CH2)7NH2-O-H
B)CH3(CH2)7NH2-Cl
C)CH3(CH2)7NH-CH3
D)CH3(CH2)7NH3+ Cl-
E)CH3(CH2)7NH2-O-CH3
A)CH3(CH2)7NH2-O-H
B)CH3(CH2)7NH2-Cl
C)CH3(CH2)7NH-CH3
D)CH3(CH2)7NH3+ Cl-
E)CH3(CH2)7NH2-O-CH3

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
72
The compound below has which functional groups? 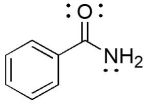
A)aromatic and carboxylic acid
B)aromatic and amide
C)aromatic and amine
D)aromatic and alcohol
E)aromatic and aldehyde

A)aromatic and carboxylic acid
B)aromatic and amide
C)aromatic and amine
D)aromatic and alcohol
E)aromatic and aldehyde

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
73
The compound below has which functional groups? 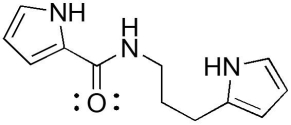
A)ester and amide
B)amide and amine
C)aldehyde and amine
D)carboxylic acid and amine
E)ester and amine

A)ester and amide
B)amide and amine
C)aldehyde and amine
D)carboxylic acid and amine
E)ester and amine

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
74
The compound below is which type of compound? 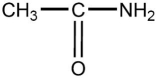
A)aldehyde
B)amine
C)ester
D)amide
E)amino acid

A)aldehyde
B)amine
C)ester
D)amide
E)amino acid

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
75
The compound below is named 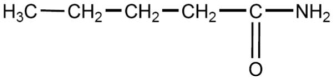
A)pentanamine.
B)pentamine.
C)1-pentanamide.
D)pentanamide.
E)pentaketoneamine.
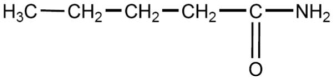
A)pentanamine.
B)pentamine.
C)1-pentanamide.
D)pentanamide.
E)pentaketoneamine.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
76
For the following segment of a polymer, what would be the expected monomer used to make it? 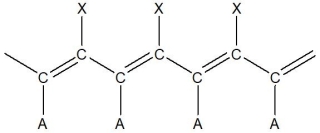
A)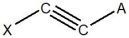
B)HC=CH
C)
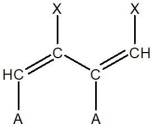
D)
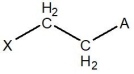
E)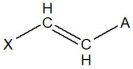

A)

B)HC=CH
C)

D)

E)


Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
77
Polymers formed by the addition of monomer units by an initiator are often called
A)unit polymers.
B)chain-growth polymers.
C)branching polymers.
D)condensation polymers.
E)background polymers.
A)unit polymers.
B)chain-growth polymers.
C)branching polymers.
D)condensation polymers.
E)background polymers.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
78
A polymer is formed by the removal of water from the monomer mixture are often called
A)unit polymers.
B)chain-growth polymers.
C)branching polymers.
D)condensation polymers.
E)background polymers.
A)unit polymers.
B)chain-growth polymers.
C)branching polymers.
D)condensation polymers.
E)background polymers.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following is a true statement with respect to molecular mass and strength?
A)High molecular mass polymers tend to be gases.
B)High molecular mass polymers tend to have low strength.
C)Low molecular mass polymers tend to have low strength.
D)All polymers have high strength and molecular mass.
E)Molecular mass tends to have no effect on polymer strength.
A)High molecular mass polymers tend to be gases.
B)High molecular mass polymers tend to have low strength.
C)Low molecular mass polymers tend to have low strength.
D)All polymers have high strength and molecular mass.
E)Molecular mass tends to have no effect on polymer strength.

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck
80
Which functional group is not usually found in carbohydrates?
A)aldehyde
B)amide
C)ether
D)hydroxy
E)ketone
A)aldehyde
B)amide
C)ether
D)hydroxy
E)ketone

Unlock Deck
Unlock for access to all 140 flashcards in this deck.
Unlock Deck
k this deck


