Deck 22: Metal Complexes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/113
Play
Full screen (f)
Deck 22: Metal Complexes
1
Which statement below about ligands that form coordination complexes with transition metals is false?
A)A ligand that attaches itself to a metal ion is acting as a Lewis acid.
B)Neutral molecules can function as ligands and can form coordination complexes with transition metal ions.
C)Anions can function as ligands and can form coordination complexes with transition metal ions.
D)The ligand in a coordination complex contains a particular atom which functions as the donor atom.
E)The bond between the ligand and the transition metal ion in a coordination complex is a coordinate covalent bond.
A)A ligand that attaches itself to a metal ion is acting as a Lewis acid.
B)Neutral molecules can function as ligands and can form coordination complexes with transition metal ions.
C)Anions can function as ligands and can form coordination complexes with transition metal ions.
D)The ligand in a coordination complex contains a particular atom which functions as the donor atom.
E)The bond between the ligand and the transition metal ion in a coordination complex is a coordinate covalent bond.
A ligand that attaches itself to a metal ion is acting as a Lewis acid.
2
Which of the following is true of all ligands?
A)Ligands can only form one bond with a metal ion.
B)Stronger ligands have a larger diameter.
C)Ligands must contain elements that have a very low electronegativity.
D)Ligands act as a Lewis base when they attach to a metal ion.
E)Ligands are electron deficient.
A)Ligands can only form one bond with a metal ion.
B)Stronger ligands have a larger diameter.
C)Ligands must contain elements that have a very low electronegativity.
D)Ligands act as a Lewis base when they attach to a metal ion.
E)Ligands are electron deficient.
Ligands act as a Lewis base when they attach to a metal ion.
3
The number of possible donor atoms in the EDTA4- (ethylenediaminetetraacetate)ligand is:
A)2
B)3
C)4
D)6
E)8
A)2
B)3
C)4
D)6
E)8
6
4
The number of possible donor atoms in each bidentate ligand, C2O42-, in the [Fe(C2O4)3]3- coordination complex ion is:
A)2
B)3
C)4
D)6
E)8
A)2
B)3
C)4
D)6
E)8

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
5
The charge on the metal ion in the coordination complex ion, [Co(CO3)3]3, is:
A)0
B)2+
C)3
D)3+
E)6
A)0
B)2+
C)3
D)3+
E)6

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
6
A coordination compound formed from cobalt(III)sulfate and ammonia contains six ammonia molecules as ligands. The formula of the compound is
A)Co2(SO4)3.6NH3
B)[Co(NH3)6]2(SO4)3
C)Co3SO4(NH3)6
D)Co2(SO4)3(NH4)6
E)Co2[(NH3)6](SO4)3
A)Co2(SO4)3.6NH3
B)[Co(NH3)6]2(SO4)3
C)Co3SO4(NH3)6
D)Co2(SO4)3(NH4)6
E)Co2[(NH3)6](SO4)3

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
7
A coordination compound formed from chromium(III)chloride contains five ammonia molecules and one water molecule, which function as ligands. The coordination compound's formula is:
A)[Cr(NH3)5(H2O)]Cl3
B)Cr3Cl(NH3)5H2O
C)CrCl3.5NH3.H2O
D)Cr(NH3)5Cl3.H2O
E)[Cr(NH3)5]Cl3.H2O
A)[Cr(NH3)5(H2O)]Cl3
B)Cr3Cl(NH3)5H2O
C)CrCl3.5NH3.H2O
D)Cr(NH3)5Cl3.H2O
E)[Cr(NH3)5]Cl3.H2O

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
8
A compound contains a transition metal coordination complex. This coordination complex consists of one iron(III)ion with three C2O42- ions attached as ligands. Which formula below describes a compound that fits this description?
A)K3[Fe(C2O4)3]
B)Fe(C2O4)3
C)[Fe(C2O4)3]Cl3
D)Fe(C2O4)3](SO4)3
E)Ca3[Fe(C2O4)3]
A)K3[Fe(C2O4)3]
B)Fe(C2O4)3
C)[Fe(C2O4)3]Cl3
D)Fe(C2O4)3](SO4)3
E)Ca3[Fe(C2O4)3]

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
9
A complex ion is formed by a Co3+ ion, which is linked to one carbonate ion and four ammonia molecules. Which formula correctly represents a salt of this complex ion?
A)Na3[Co(NH3)4CO3]
B)Na[Co(NH3)4CO3]
C)[Co(NH3)4CO3]CO3
D)[Co(NH3)4CO3]Cl2
E)[Co(NH3)4CO3]2SO4
A)Na3[Co(NH3)4CO3]
B)Na[Co(NH3)4CO3]
C)[Co(NH3)4CO3]CO3
D)[Co(NH3)4CO3]Cl2
E)[Co(NH3)4CO3]2SO4

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
10
A compound contains a transition metal coordination complex. This coordination complex consists of one cobalt(III)ion with five ammonia molecules and one cyanide ion attached as ligands. Which formula correctly describes a compound that would fit the description?
A)K4[CoCN(NH3)5]
B)K2[CoCN(NH3)5]
C)[CoCN(NH3)5](SO4)2
D)[CoCN(NH3)5]SO4
E)[CoCN(NH3)5]Cl3
A)K4[CoCN(NH3)5]
B)K2[CoCN(NH3)5]
C)[CoCN(NH3)5](SO4)2
D)[CoCN(NH3)5]SO4
E)[CoCN(NH3)5]Cl3

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
11
A compound contains a transition metal coordination complex. This coordination complex consists of one iron(III)ion with 3 ammonia molecules, one ethylenediamine (en)molecule, and one cyanide ion attached as ligands. Which formula correctly describes a compound that would fit the description?
A)K4[FeCN(NH3)3(en)2]
B)K2[FeCN(NH3)3(en)]
C)[Fe(CN)2(NH3)5(en)2](SO4)2
D)[FeCN(NH3)3(en)2]SO4
E)[Fe(en)CN(NH3)3]Cl3
A)K4[FeCN(NH3)3(en)2]
B)K2[FeCN(NH3)3(en)]
C)[Fe(CN)2(NH3)5(en)2](SO4)2
D)[FeCN(NH3)3(en)2]SO4
E)[Fe(en)CN(NH3)3]Cl3

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
12
Which coordination complex would you expect to be the most stable?
A)Co(NH3)63+
B)CoF63
C)Co(en)33+
D)Co(dien)23+ dien = H2N-CH2-CH2-NH-CH2-CH2-NH2
E)Co(EDTA)EDTA = ethylenediaminetetraacetate
A)Co(NH3)63+
B)CoF63
C)Co(en)33+
D)Co(dien)23+ dien = H2N-CH2-CH2-NH-CH2-CH2-NH2
E)Co(EDTA)EDTA = ethylenediaminetetraacetate

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following ligands would be the most difficult to remove from an iron(III)cation under similar conditions?
A)NH3
B)F
C)en
D)O2
E)EDTA4-
A)NH3
B)F
C)en
D)O2
E)EDTA4-

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
14
EDTA4- (ethylenediaminetetraacetate anion), shown below, is capable of acting as a ________ ligand. 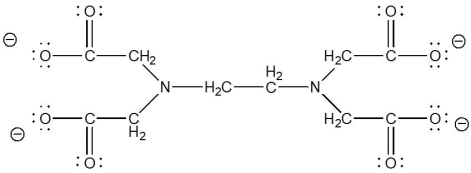
A)monodentate
B)bidentate
C)tridentate
D)tetradentate
E)polydentate

A)monodentate
B)bidentate
C)tridentate
D)tetradentate
E)polydentate

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
15
How many donor atoms are on a monodentate ligand?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
16
How many donor atoms are on a bidentate ligand?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
17
How many bonds can a polydentate ligand form with a metal cation?
A)0
B)1
C)2
D)4
E)both C and D are possible
A)0
B)1
C)2
D)4
E)both C and D are possible

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
18
Which species can function as a chelating ligand?
A)NH3
B)NH4+
C)CN-
D)CH3-CH2-CH2-NH2
E)NH2-CH2-CH2-NH2
A)NH3
B)NH4+
C)CN-
D)CH3-CH2-CH2-NH2
E)NH2-CH2-CH2-NH2

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
19
Which species can function as a chelating ligand?
A)NH3
B)NH4+
C)CN-
D)CH3-CH2-CH2-O-H
E)NH2-CH2-CH2-NH-CH3
A)NH3
B)NH4+
C)CN-
D)CH3-CH2-CH2-O-H
E)NH2-CH2-CH2-NH-CH3

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
20
Which cobalt complex should be the most stable (having the largest formation constant)?
A)Co(NH3)62+
B)Co(NH3)63+
C)Co(H2O)62+
D)Co(F)63-
E)Co(en)33+
A)Co(NH3)62+
B)Co(NH3)63+
C)Co(H2O)62+
D)Co(F)63-
E)Co(en)33+

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
21
The name for the complex ion, [Ni(CN)4]2, is:
A)tetracyanonickel(II)
B)nickel(II)tetracyanide
C)tetracyanonickelo(II)
D)tetracyanonickelate(II)
E)tetracyanonickelite(II)
A)tetracyanonickel(II)
B)nickel(II)tetracyanide
C)tetracyanonickelo(II)
D)tetracyanonickelate(II)
E)tetracyanonickelite(II)

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
22
The name for the complex ion, [Co(NH3)6]3+, is
A)cobalt hexammine.
B)cobalt(III)hexamine.
C)hexamminecobaltate(III).
D)hexaminocobaltate(III).
E)hexamminecobalt(III).
A)cobalt hexammine.
B)cobalt(III)hexamine.
C)hexamminecobaltate(III).
D)hexaminocobaltate(III).
E)hexamminecobalt(III).

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
23
The name for the transition metal complex [Fe(CN)6]3- is
A)hexacyano ferrate(III).
B)hexacyanoiron(II).
C)iron(III)hexacyanide.
D)ferrous cyanide.
E)ferric cyanide.
A)hexacyano ferrate(III).
B)hexacyanoiron(II).
C)iron(III)hexacyanide.
D)ferrous cyanide.
E)ferric cyanide.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
24
The name for the transition metal complex [Cr(H2O)6]Cl3 is
A)hexawaterchromium(III)chloride.
B)hexaaquachromium(III)trichloride.
C)hexaaquachromium(III)chloride.
D)chromium(III)aqua chloride.
E)chromium(III)chloride hexahydrate.
A)hexawaterchromium(III)chloride.
B)hexaaquachromium(III)trichloride.
C)hexaaquachromium(III)chloride.
D)chromium(III)aqua chloride.
E)chromium(III)chloride hexahydrate.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
25
The correct name for the transition metal complex [CoCl3(NH3)3] is
A)cobalt(III)chloride triammine.
B)triamminetrichlorocobalt(III).
C)triamminetrichlorocobalt(II).
D)cobalt ammonia chloride.
E)cobalt(III)ammonium chloride.
A)cobalt(III)chloride triammine.
B)triamminetrichlorocobalt(III).
C)triamminetrichlorocobalt(II).
D)cobalt ammonia chloride.
E)cobalt(III)ammonium chloride.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
26
The name for the complex ion, [AuCl4], is
A)gold tetrachloride.
B)gold(II)tetrachloride.
C)auric tetrachloride.
D)tetrachlorogold(III).
E)tetrachloroaurate(III).
A)gold tetrachloride.
B)gold(II)tetrachloride.
C)auric tetrachloride.
D)tetrachlorogold(III).
E)tetrachloroaurate(III).

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
27
The correct name for the compound, [CrCl2(en)2]2C2O4, is
A)dichlorobis(ethylenediamine)chromium(III)oxalate.
B)bis(ethylenediamine)dichlorochromate(III)oxalate.
C)dichlorobis(ethylenediamine)oxalatochromium(III).
D)dichlorobis(ethylenediamine)oxalatochromate(III).
E)bis(dichlorobis(ethylenediamine)chromium(III))oxalate.
A)dichlorobis(ethylenediamine)chromium(III)oxalate.
B)bis(ethylenediamine)dichlorochromate(III)oxalate.
C)dichlorobis(ethylenediamine)oxalatochromium(III).
D)dichlorobis(ethylenediamine)oxalatochromate(III).
E)bis(dichlorobis(ethylenediamine)chromium(III))oxalate.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
28
The compound, [Co(NH3)6]2[PtCl4]3, is named
A)cobalt(III)hexammine tetrachloroplatinate(II).
B)cobalt(II)hexammine tetrachloroplatinate(II).
C)cobalt(III)hexammine platinum(II)tetrachloride.
D)hexamminecobalt(III)tetrachloroplatinate(II).
E)hexamminecobalt(III)platinum(II)tetrachloride.
A)cobalt(III)hexammine tetrachloroplatinate(II).
B)cobalt(II)hexammine tetrachloroplatinate(II).
C)cobalt(III)hexammine platinum(II)tetrachloride.
D)hexamminecobalt(III)tetrachloroplatinate(II).
E)hexamminecobalt(III)platinum(II)tetrachloride.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
29
The compound, (NH4)3[FeF6], is named
A)ammonium hexafluoroiron(III).
B)ammonium hexafluoroferrate(III).
C)ammonium iron(III)hexafluoride.
D)hexafluoroiron(III)ammonium.
E)triammonium hexafluoroiron(III).
A)ammonium hexafluoroiron(III).
B)ammonium hexafluoroferrate(III).
C)ammonium iron(III)hexafluoride.
D)hexafluoroiron(III)ammonium.
E)triammonium hexafluoroiron(III).

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
30
The coordination complex, [Co(NO2)(NH3)5]Cl2, is named
A)pentaamminenitrocobalt(III)chloride.
B)pentaamminenitritocobalt(II)chloride.
C)pentaamminenitrocobalt(II)chloride.
D)pentaamminenitritocobalt(III)chloride.
E)nitropentaamminecobalt(II)chloride.
A)pentaamminenitrocobalt(III)chloride.
B)pentaamminenitritocobalt(II)chloride.
C)pentaamminenitrocobalt(II)chloride.
D)pentaamminenitritocobalt(III)chloride.
E)nitropentaamminecobalt(II)chloride.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
31
The coordination complex, [Co(NO2)(NH3)5]Cl2, is named
A)pentaamminenitrocobalt(III)chloride.
B)pentaamminenitritocobalt(II)chloride.
C)pentaamminenitrocobalt(II)chloride.
D)pentaamminenitritocobalt(III)chloride.
E)nitropentaamminecobalt(II)chloride.
A)pentaamminenitrocobalt(III)chloride.
B)pentaamminenitritocobalt(II)chloride.
C)pentaamminenitrocobalt(II)chloride.
D)pentaamminenitritocobalt(III)chloride.
E)nitropentaamminecobalt(II)chloride.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
32
The metal complex, [Cr(en)3]Cl3, is named
A)tri(ethylenediamine)chromium(III)trichloride.
B)tri(ethylenediamine)chromium(III)chloride.
C)trichloro ethylenediaminechromate(III).
D)tris(ethylenediamine)chromium(III)chloride.
E)bis(ethylenediamine)chromium(III)trichloride.
A)tri(ethylenediamine)chromium(III)trichloride.
B)tri(ethylenediamine)chromium(III)chloride.
C)trichloro ethylenediaminechromate(III).
D)tris(ethylenediamine)chromium(III)chloride.
E)bis(ethylenediamine)chromium(III)trichloride.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
33
The complex ion, [Cr(dien)2]3+ is shown below. The coordination number of the chromium ion in the [Cr(dien)2]3+ is: dien = ![<strong>The complex ion, [Cr(dien)<sub>2</sub>]<sup>3+ </sup>is shown below. The coordination number of the chromium ion in the [Cr(dien)<sub>2</sub>]<sup>3+</sup> is: dien = </strong> A)1 B)2 C)3 D)4 E)6](https://storage.examlex.com/TBW1039/11ee6e7b_ba52_df53_bf7f_87d97d51d4c3_TBW1039_00.jpg)
A)1
B)2
C)3
D)4
E)6
![<strong>The complex ion, [Cr(dien)<sub>2</sub>]<sup>3+ </sup>is shown below. The coordination number of the chromium ion in the [Cr(dien)<sub>2</sub>]<sup>3+</sup> is: dien = </strong> A)1 B)2 C)3 D)4 E)6](https://storage.examlex.com/TBW1039/11ee6e7b_ba52_df53_bf7f_87d97d51d4c3_TBW1039_00.jpg)
A)1
B)2
C)3
D)4
E)6

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
34
The coordination number of Ag, in the following complex, is: 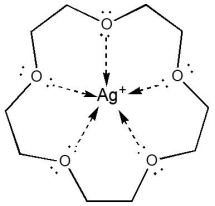
A)1
B)2
C)3
D)4
E)5

A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
35
The coordination number of platinum in the complex ion, [Pt(C2O4)2]2, is:
A)1
B)2
C)3
D)4
E)6
A)1
B)2
C)3
D)4
E)6

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
36
The coordination number of platinum in the complex ion in the compound, [Pt(NH3)4]SO4, is:
A)1
B)2
C)3
D)4
E)6
A)1
B)2
C)3
D)4
E)6

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
37
The coordination number of the cobalt ion in the coordination complex ion present in [Co(Cl)(NH3)4(H2O)]Cl2, is:
A)3
B)4
C)5
D)6
E)8
A)3
B)4
C)5
D)6
E)8

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
38
The coordination number of the nickel ion in the complex ion, 
A)1
B)2
C)3
D)4
E)6
A)1
B)2
C)3
D)4
E)6

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
39
The coordination number of the cobalt ion in the complex ion in the [Co(Cl)(NH3)5]Cl2 compound is:
A)1
B)3
C)5
D)6
E)8
A)1
B)3
C)5
D)6
E)8

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
40
Cu2+ ion reacts with ammonia molecules to form the Cu(NH3)42+ ion.Which statement below is true?
A)The copper ion is functioning as a Lewis base.
B)The ammonia molecule is functioning as an acceptor molecule.
C)The bond between the ammonia and the copper(II)ion is an ionic bond.
D)The ammonia molecule is a monodentate ligand.
E)The copper(II)ion is a 6-coordinate species in this complex ion.
A)The copper ion is functioning as a Lewis base.
B)The ammonia molecule is functioning as an acceptor molecule.
C)The bond between the ammonia and the copper(II)ion is an ionic bond.
D)The ammonia molecule is a monodentate ligand.
E)The copper(II)ion is a 6-coordinate species in this complex ion.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
41
The correct name of the neutral coordination complex shown below is 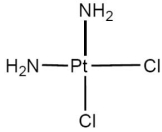
A)cisdiaminodichloroplatinate(II).
B)cisdiaminodichloroplatinum(II).
C)cisdiamminedichloroplatinum(II).
D)transdiaminodichloroplatinum(II).
E)transdiamminedichloroplatinum(II).

A)cisdiaminodichloroplatinate(II).
B)cisdiaminodichloroplatinum(II).
C)cisdiamminedichloroplatinum(II).
D)transdiaminodichloroplatinum(II).
E)transdiamminedichloroplatinum(II).

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
42
A platinum coordination complex has the formula, [PtCl(NH3)2Br], and exhibits a square planar geometry. How many geometric isomers of this compound should exist?
A)1 (no isomers)
B)2
C)3
D)4
E)5
A)1 (no isomers)
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
43
A platinum coordination complex has the formula, [Pt(Br)(Cl)(I)(NH3 )], and exhibits a square planar geometry. How many geometric isomers of this compound should exist?
A)1 (no isomers)
B)2
C)3
D)4
E)5
A)1 (no isomers)
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
44
A platinum coordination complex has the formula, [PtBr(CN)3]+, and exhibits a square planar geometry. How many geometric isomers of this compound should exist?
A)1 (no isomers)
B)2
C)3
D)4
E)5
A)1 (no isomers)
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
45
A coordination complex in which the ligands exhibit an octahedral geometry about the central metal ion has the formula, [CoCl2(NH3)4]. How many geometric isomers of this compound should exist?
A)1 (no isomers)
B)2
C)3
D)4
E)5
A)1 (no isomers)
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
46
A coordination complex in which the ligands exhibit an octahedral geometry about the central metal ion has the formula, [CoCl3(NH3)3]-. How many geometric isomers of this compound should exist?
A)1 (no isomers)
B)2
C)3
D)4
E)5
A)1 (no isomers)
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
47
Which complex ion is a chiral isomer?
A)square planar [Pt(en)2]2+
B)tetrahedral [NiCl4]2
C)octahedral [Co(en)3]3+
D)octahedral [Co(NH3)4Cl2]+
E)square planar [Pt(NH3)ClBrI]
A)square planar [Pt(en)2]2+
B)tetrahedral [NiCl4]2
C)octahedral [Co(en)3]3+
D)octahedral [Co(NH3)4Cl2]+
E)square planar [Pt(NH3)ClBrI]

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
48
A coordination complex in which the ligands exhibit an octahedral geometry about the central metal ion has the formula, [CrCl2(en)2]+. How many geometric isomers of this compound should exist?
A)1 (no isomers)
B)2
C)3
D)4
E)5
A)1 (no isomers)
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
49
A coordination complex in which the ligands exhibit an octahedral geometry about the central metal ion has the formula, [CrCl2(en)2]+. How many isomers of this complex ion should exist?
A)1 (no isomers)
B)2
C)3
D)4
E)5
A)1 (no isomers)
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
50
The splitting of the d-orbitals of transition metals by a particular complex geometry gives rise to the pattern: ? 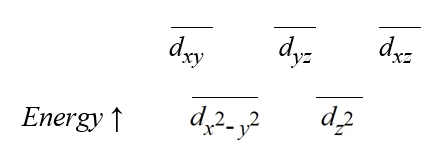 Which one of the following geometries produces the splitting pattern seen above?
Which one of the following geometries produces the splitting pattern seen above?
A)linear
B)octahedral
C)planar triangular
D)square planar
E)tetrahedral
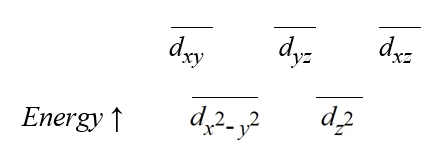 Which one of the following geometries produces the splitting pattern seen above?
Which one of the following geometries produces the splitting pattern seen above?A)linear
B)octahedral
C)planar triangular
D)square planar
E)tetrahedral

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
51
A complex ion which has its absorption maximum in the blue region of the visible spectrum would appear to be ________ in color.
A)blue-green
B)green-yellow
C)blue-violet
D)orange-yellow
E)red-violet
A)blue-green
B)green-yellow
C)blue-violet
D)orange-yellow
E)red-violet

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
52
A complex ion which has its absorption maximum in the red region of the visible spectrum would appear to be ________ in color.
A)green-blue
B)yellow-green
C)orange-yellow
D)blue-violet
E)red-violet
A)green-blue
B)yellow-green
C)orange-yellow
D)blue-violet
E)red-violet

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
53
Many coordination complexes are colored due to absorption of light in the visible region of the spectrum. Which one of the following coordination complexes of transition metals is not expected to be a colored complex?Hint: Electrons transitioning between occupied and unoccupied d-orbitals produce colors in metal complexes.
A)Co(NH3)63+
B)Cr(NH3)62+
C)Cu(NH3)42+
D)V(H2O)63+
E)Zn(NH3)42+
A)Co(NH3)63+
B)Cr(NH3)62+
C)Cu(NH3)42+
D)V(H2O)63+
E)Zn(NH3)42+

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
54
The following order is observed in the spectrochemical series for ligands CN- > NO2- > en > NH3 > H2O > C2O42- > OH- > F- > Cl- > Br- > I- Based on this series, which of the following would absorb the highest energy light?
A)[Fe(NH3)6]2+
B)[Fe(NH3)6]3+
C)[Os(CN)6]4-
D)[Os(NH3)6]2+
E)[Fe(CN)6]3-
A)[Fe(NH3)6]2+
B)[Fe(NH3)6]3+
C)[Os(CN)6]4-
D)[Os(NH3)6]2+
E)[Fe(CN)6]3-

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
55
The following order is observed in the spectrochemical series for ligands CN- > NO2- > en > NH3 > H2O > C2O42- > OH- > F- > Cl- > Br- > I- Based on this series, which of the following would absorb the highest energy light?
A)[Os(NH3)6]2+
B)[Os(NH3)6]4+
C)[Os(H2O)6]4+
D)[Os(H2O)6]2+
E)[Os(Br)6]3-
A)[Os(NH3)6]2+
B)[Os(NH3)6]4+
C)[Os(H2O)6]4+
D)[Os(H2O)6]2+
E)[Os(Br)6]3-

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
56
A Mo2+ ion has ________ 4d electrons.
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
57
The following order is observed in the spectrochemical series for ligands CN- > NO2- > en > NH3 > H2O > C2O42- > OH- > F- > Cl- > Br- > I- It was observed that for the two 6-coordinate cobalt complexes, CoF63 and Co(NH3)63+, one is diamagnetic and one is paramagnetic. How many unpaired electrons are there in the paramagnetic complex ion?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
58
The following order is observed in the spectrochemical series for ligands CN- > NO2- > en > NH3 > H2O > C2O42- > OH- > F- > Cl- > Br- > I- How many unpaired electrons would you expect to find in the complex ion, Cr(CN)63 if the value of the splitting parameter is as large as it is in typical cyanide complexes?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
59
The following order is observed in the spectrochemical series for ligands CN- > NO2- > en > NH3 > H2O > C2O42- > OH- > F- > Cl- > Br- > I-Cr(NH3)63+ and Ni(NH3)62+ are both positively charged coordination complexes. Which statement describes the magnetic properties of the complexes?
A)Cr(NH3)63+ and Ni(NH3)62+ are both diamagnetic.
B)Cr(NH3)63+ is diamagnetic while Ni(NH3)62+ is paramagnetic.
C)Cr(NH3)63+ is paramagnetic while Ni(NH3)62+ is diamagnetic.
D)Both complex ions are paramagnetic, but Cr(NH3)63+ has a larger number of unpaired electrons than Ni(NH3)62+ hence its paramagnetism is more pronounced.
E)Both complex ions are paramagnetic, but Ni(NH3)62+ has a larger number of unpaired electrons than Cr(NH3)63+ hence its paramagnetism is more pronounced.
A)Cr(NH3)63+ and Ni(NH3)62+ are both diamagnetic.
B)Cr(NH3)63+ is diamagnetic while Ni(NH3)62+ is paramagnetic.
C)Cr(NH3)63+ is paramagnetic while Ni(NH3)62+ is diamagnetic.
D)Both complex ions are paramagnetic, but Cr(NH3)63+ has a larger number of unpaired electrons than Ni(NH3)62+ hence its paramagnetism is more pronounced.
E)Both complex ions are paramagnetic, but Ni(NH3)62+ has a larger number of unpaired electrons than Cr(NH3)63+ hence its paramagnetism is more pronounced.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
60
When bound to oxygen, the iron(II)found in a heme group has what coordination number?
A)2
B)4
C)5
D)6
E)8
A)2
B)4
C)5
D)6
E)8

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
61
The heme found in cyanocobalamin, otherwise known as vitamin B12, contains what metal ion?
A)Fe(II)
B)Fe(III)
C)Co(II)
D)Cr(III)
E)Cu(II)
A)Fe(II)
B)Fe(III)
C)Co(II)
D)Cr(III)
E)Cu(II)

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
62
Heme groups contain a porphyrin structure as a part of their structure. The donor atoms in a porphyrin structure are composed of what element?
A)Oxygen
B)Nitrogen
C)Carbon
D)Iron
E)Sulfur
A)Oxygen
B)Nitrogen
C)Carbon
D)Iron
E)Sulfur

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
63
The iron found in hemoglobin serves what purpose?
A)It holds together the structure.
B)It oxidizes bacteria found in the blood, killing them.
C)It converts oxygen into carbon dioxide.
D)It bonds to oxygen and allows the blood to carry oxygen throughout the body.
E)It allows hemoglobin to bond to DNA, helping to protect the DNA from damage.
A)It holds together the structure.
B)It oxidizes bacteria found in the blood, killing them.
C)It converts oxygen into carbon dioxide.
D)It bonds to oxygen and allows the blood to carry oxygen throughout the body.
E)It allows hemoglobin to bond to DNA, helping to protect the DNA from damage.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
64
A bidentate ligand has ________ donor atom(s).

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
65
A monodentate ligand has ________ donor atom(s).

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
66
Ligands act as Lewis ________ when they react with a metal cation and form a bond.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
67
The equilibrium constant expression obtained for the process
Cu2+(aq)+ 4NH3(aq)![The equilibrium constant expression obtained for the process Cu<sup>2+</sup>(aq)+ 4NH<sub>3</sub>(aq) Cu(NH<sub>3</sub>)<sup>2+</sup>(aq)is[Cu(NH<sub>3</sub>)<sub>4</sub><sup>2+</sup>]/[Cu<sup>2+</sup>][NH<sub>3</sub>]<sup>4</sup>. This expression is called the ________ constant.](https://storage.examlex.com/TBW1039/11ee6e7b_ba53_c9b7_bf7f_9d1bbbb1c0b3_TBW1039_11.jpg) Cu(NH3)2+(aq)is[Cu(NH3)42+]/[Cu2+][NH3]4. This expression is called the ________ constant.
Cu(NH3)2+(aq)is[Cu(NH3)42+]/[Cu2+][NH3]4. This expression is called the ________ constant.
Cu2+(aq)+ 4NH3(aq)
![The equilibrium constant expression obtained for the process Cu<sup>2+</sup>(aq)+ 4NH<sub>3</sub>(aq) Cu(NH<sub>3</sub>)<sup>2+</sup>(aq)is[Cu(NH<sub>3</sub>)<sub>4</sub><sup>2+</sup>]/[Cu<sup>2+</sup>][NH<sub>3</sub>]<sup>4</sup>. This expression is called the ________ constant.](https://storage.examlex.com/TBW1039/11ee6e7b_ba53_c9b7_bf7f_9d1bbbb1c0b3_TBW1039_11.jpg) Cu(NH3)2+(aq)is[Cu(NH3)42+]/[Cu2+][NH3]4. This expression is called the ________ constant.
Cu(NH3)2+(aq)is[Cu(NH3)42+]/[Cu2+][NH3]4. This expression is called the ________ constant.
Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
68
A compound contains a transition metal coordination complex. This coordination complex consists of one Ni(II)ion with 2 water molecules, two ethylenediamine (en)molecules, and the remaining positions filled with Cl ions acting as ligands. Write the correct formula for this complex ion with the correct charge.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
69
A compound contains a transition metal coordination complex. This coordination complex consists of one Cu(I)ion, complexed with two water molecules, one ethylenediamine molecule, and Cl ions in the remaining ligand positions. Write the correct formula for this complex ion, including the correct charge.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
70
Write the correct formula for the metal complex with the name potassium hexacyanoferrate(III).

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
71
Write the correct formula for the metal complex with the name ammonium diaquatetrachlorocobaltate(II).

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
72
Write the correct name for the metal complex Na[Co(NO2)6].

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
73
Write the correct name for the metal complex [Pt(en)2Cl2](NO3)2.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
74
The correct formula for the dichlorobis(ethylenediamine)chromium(III)ion is ________.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
75
The ethylenediamine molecule (en), functions as a bidentate ligand and forms a coordination complex with platinum: [Pt(en)2]2+. What is the coordination number to the platinum(II)ion in this coordination complex?

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
76
The ethylenediamine molecule (en), functions as a bidentate ligand and forms a coordination complex with cobalt: [Co(en)2(H2O)2]3+. Assign a coordination number and a charge to the cobalt ion in this coordination complex.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
77
Give the names of both isomers of Pt(NH3)2Br2.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
78
In the coordination complex [Ag(NH3)2]Br, the coordination number and oxidation number of the central atom are ________.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
79
In the coordination complex [Pt(NH3)2Cl2], the coordination number and oxidation number of the central atom are ________.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck
80
In the coordination complex [Co(NH3)2(H2O)2Cl2]+, the coordination number and oxidation number of the central atom are ________.

Unlock Deck
Unlock for access to all 113 flashcards in this deck.
Unlock Deck
k this deck


