Deck 19: Thermodynamics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/109
Play
Full screen (f)
Deck 19: Thermodynamics
1
The mathematical equation that expresses the first law of thermodynamics is
A) H = E + p V.
B) H = E - p V.
C) H = q + w.
D) E = q + w.
E) H = q + E.
A) H = E + p V.
B) H = E - p V.
C) H = q + w.
D) E = q + w.
E) H = q + E.
E = q + w.
2
The standard enthalpy of reaction, H°rxn for C2H2(g)+ 2 H2(g) C2H6(g)is -311.5 kJ mol-1. Determine the value of E°rxn for this reaction.
A)-306.5 kJ mol-1
B)-309.0 kJ mol-1
C)-314.0 kJ mol-1
D)-316.46 kJ mol-1
E)+4646 kJ mol-1
A)-306.5 kJ mol-1
B)-309.0 kJ mol-1
C)-314.0 kJ mol-1
D)-316.46 kJ mol-1
E)+4646 kJ mol-1
-306.5 kJ mol-1
3
The standard enthalpy of reaction, H° at 25 C° NH3(g)+ HCl(g) NH4Cl(s)is -175.9 kJ mol-1. Determine the value of E°rxn for this reaction.
A)-164.8 kJ mol-1
B)-170.9 kJ mol-1
C)-173.4 kJ mol-1
D)-180.9 kJ mol-1
E)+5134 kJ mol-1
A)-164.8 kJ mol-1
B)-170.9 kJ mol-1
C)-173.4 kJ mol-1
D)-180.9 kJ mol-1
E)+5134 kJ mol-1
-170.9 kJ mol-1
4
The standard enthalpy of reaction, H°rxn, for the reaction,C4H10(g)+ 13/2 O2(g) 4 CO2(g)+ 5 H2O(l)is -2877 kJ mol-1. Determine the value of E°rxn for this reaction.
A)-2868 kJ mol-1
B)-2871 kJ mol-1
C)-2880 kJ mol-1
D)-2886 kJ mol-1
E)+2886 kJ mol-1
A)-2868 kJ mol-1
B)-2871 kJ mol-1
C)-2880 kJ mol-1
D)-2886 kJ mol-1
E)+2886 kJ mol-1

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
5
The standard enthalpy of reaction, H°rxn, for the reaction,CaO(s)+ SO3(g) CaSO4(s)is -401.5 kJ mol-1. Determine the value of E°rxn for this reaction.
A)-362.2 kJ mol-1
B)-399.0 kJ mol-1
C)-404.0 kJ mol-1
D)-2880 kJ mol-1
E)+2077 kJ mol-1
A)-362.2 kJ mol-1
B)-399.0 kJ mol-1
C)-404.0 kJ mol-1
D)-2880 kJ mol-1
E)+2077 kJ mol-1

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
6
Which statement is true?
A)Spontaneous changes are always accompanied by an increase in the entropy of the system.
B)Spontaneous changes are always accompanied by a decrease in the entropy of the system.
C)Spontaneous changes are always accompanied by an increase in the enthalpy of the system.
D)Spontaneous changes are always accompanied by a decrease in the enthalpy of the system.
E)Most highly exothermic chemical reactions are also spontaneous chemical reactions.
A)Spontaneous changes are always accompanied by an increase in the entropy of the system.
B)Spontaneous changes are always accompanied by a decrease in the entropy of the system.
C)Spontaneous changes are always accompanied by an increase in the enthalpy of the system.
D)Spontaneous changes are always accompanied by a decrease in the enthalpy of the system.
E)Most highly exothermic chemical reactions are also spontaneous chemical reactions.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
7
Which process is accompanied by an increase in entropy?
A)setting up a stack of dominos
B)setting up decorations on a Christmas tree
C)filing correspondence in file folders and placing them in hanging file folders
D)dropping a glass pane on the front walk of your residence
E)restocking a canned goods shelf display in a supermarket
A)setting up a stack of dominos
B)setting up decorations on a Christmas tree
C)filing correspondence in file folders and placing them in hanging file folders
D)dropping a glass pane on the front walk of your residence
E)restocking a canned goods shelf display in a supermarket

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
8
Which reaction is accompanied by an increase in entropy?
A)ZnS(s)+ 3/2 O2(g) ZnO(s)+ SO2(g)
B)CH4(g)+ H2O(g) CO(g)+ 3H2(g)
C)BaO(s)+ CO2(g) BaCO3(s)
D)Na2CO3(s)+ CO2(g)+ H2O(g) 2 NaHCO3(s)
E)N2(g)+ 3 H2(g) 2 NH3(g)
A)ZnS(s)+ 3/2 O2(g) ZnO(s)+ SO2(g)
B)CH4(g)+ H2O(g) CO(g)+ 3H2(g)
C)BaO(s)+ CO2(g) BaCO3(s)
D)Na2CO3(s)+ CO2(g)+ H2O(g) 2 NaHCO3(s)
E)N2(g)+ 3 H2(g) 2 NH3(g)

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
9
Which reaction is accompanied by an increase in entropy?
A)2 H(g) H2(g)
B)NiCl2(s)+ 6 NH3(g) NiCl2?6NH3(s)
C)I2(g) 2 I(g)
D)ZnO(s)+ CO2(g) ZnCO3(s)
E)C2H4(g)+ Cl2(g) C2H4Cl2(l)
A)2 H(g) H2(g)
B)NiCl2(s)+ 6 NH3(g) NiCl2?6NH3(s)
C)I2(g) 2 I(g)
D)ZnO(s)+ CO2(g) ZnCO3(s)
E)C2H4(g)+ Cl2(g) C2H4Cl2(l)

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
10
Which reaction is accompanied by an increase in entropy?
A)C12H20(l)+ 17 O2(g) 12 CO2(g)+ 10 H2O(l)
B)NH4Cl(s) NH3(g)+ HCl(g)
C)2 C2H2(g)+ 5 O2(g) 4 CO2(g)+ 2 H2O(s)
D)Ba(OH)2(s)+ 2 HCl(g) BaCl2.2H2O(s)
E)(CH3)2CO(l)+ 4 O2(g) 3 CO2(g)+ 3 H2O(l)
A)C12H20(l)+ 17 O2(g) 12 CO2(g)+ 10 H2O(l)
B)NH4Cl(s) NH3(g)+ HCl(g)
C)2 C2H2(g)+ 5 O2(g) 4 CO2(g)+ 2 H2O(s)
D)Ba(OH)2(s)+ 2 HCl(g) BaCl2.2H2O(s)
E)(CH3)2CO(l)+ 4 O2(g) 3 CO2(g)+ 3 H2O(l)

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
11
Which reaction is accompanied by an increase in entropy?
A)C8H16(l)+ 12 O2(g) 8 CO2(g)+ 8 H2O(l)
B)N2(g)+ 3 H2(g) 2 NH3(g)
C)2 C2H2(g)+ 5 O2(g) 4 CO2(g)+ 2 H2O(s)
D)Ba(OH)2(s)+ CO2(g) BaCO3(s)+ H2O(l)
E)NH4NO2(s) N2(g)+ 2 H2O(l)
A)C8H16(l)+ 12 O2(g) 8 CO2(g)+ 8 H2O(l)
B)N2(g)+ 3 H2(g) 2 NH3(g)
C)2 C2H2(g)+ 5 O2(g) 4 CO2(g)+ 2 H2O(s)
D)Ba(OH)2(s)+ CO2(g) BaCO3(s)+ H2O(l)
E)NH4NO2(s) N2(g)+ 2 H2O(l)

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
12
Which process is accompanied by a decrease in the entropy of the system?
A)the mixing of one liter of water with one liter of ethylene glycol to produce one liter of solution
B)the breaking of a large rock into very many smaller pieces of crushed gravel
C)the thawing of the frozen orange juice concentrate that was left in the car
D)the spontaneous chemical reaction of TNT (a solid chemical compound)wherein it decomposes into several simple compounds, some of which are gaseous
E)the absorption of odorous gaseous compounds by the charcoal filter in your home central air cleaning unit
A)the mixing of one liter of water with one liter of ethylene glycol to produce one liter of solution
B)the breaking of a large rock into very many smaller pieces of crushed gravel
C)the thawing of the frozen orange juice concentrate that was left in the car
D)the spontaneous chemical reaction of TNT (a solid chemical compound)wherein it decomposes into several simple compounds, some of which are gaseous
E)the absorption of odorous gaseous compounds by the charcoal filter in your home central air cleaning unit

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
13
Which process is accompanied by an increase in the entropy of the system?
A)solid gold melting
B)the condensation of water on cold surface
C)the freezing of a popsicle
D)sewing a quilt
E)a cup of coffee cooling in a mug
A)solid gold melting
B)the condensation of water on cold surface
C)the freezing of a popsicle
D)sewing a quilt
E)a cup of coffee cooling in a mug

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
14
Which process is accompanied by a decrease in the entropy of the system?
A)a cup of juice spreading across the floor after being spilled
B)soaking up a chemical spill with an absorbent material
C)the sublimation of iodine crystals
D)water boiling in a steam kettle
E)butter melting for a cake
A)a cup of juice spreading across the floor after being spilled
B)soaking up a chemical spill with an absorbent material
C)the sublimation of iodine crystals
D)water boiling in a steam kettle
E)butter melting for a cake

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
15
Which of these species has the highest entropy (S°)at 25°C?
A)H2O (s)
B)H2O (l)
C)H2O (g)
A)H2O (s)
B)H2O (l)
C)H2O (g)

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
16
Which of these species has the highest entropy (S°)at 25°C?
A)CO2 (s)
B)CO2 (l)
C)CO2 (g)
A)CO2 (s)
B)CO2 (l)
C)CO2 (g)

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
17
Which statement below is always true for a spontaneous chemical reaction?
A) Ssys + Ssurr = 0
B) Ssys + Ssurr < 0
C) Ssys + Ssurr > 0
D) Ssys - Ssurr = 0
E) Ssys - Ssurr < 0
A) Ssys + Ssurr = 0
B) Ssys + Ssurr < 0
C) Ssys + Ssurr > 0
D) Ssys - Ssurr = 0
E) Ssys - Ssurr < 0

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
18
Of the species listed, which should possess the highest standard entropy (S°)for one mole of substance?
A)(CH3)2CO(l)
B)C4H10(g)
C)K2SO4(s)
D)H2O(l)
E)Br2(l)
A)(CH3)2CO(l)
B)C4H10(g)
C)K2SO4(s)
D)H2O(l)
E)Br2(l)

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
19
Which species has the highest standard entropy (S°)for one mole of substance?
A)Au(s)
B)Cd(s)
C)Hg(l)
D)Ni(s)
E)K(s)
A)Au(s)
B)Cd(s)
C)Hg(l)
D)Ni(s)
E)K(s)

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
20
Which species has the greatest standard entropy (S°)for one mole of substance?
A)I2(s)
B)Br2(l)
C)N2(l)
D)Cl2(g)
E)He(l)
A)I2(s)
B)Br2(l)
C)N2(l)
D)Cl2(g)
E)He(l)

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
21
Which species should possess the lowest standard entropy (S°)?
A)CH4(g)
B)CO2(s)
C)NH3(l)
D)H2O(l)
E)Ar(g)
A)CH4(g)
B)CO2(s)
C)NH3(l)
D)H2O(l)
E)Ar(g)

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
22
Which set has the species listed in order of increasing standard entropy, S°?
A)Au(s)< CaCO3(s)< H2O(l)
B)CaCO3(s)< H2O(l)< Au(s)
C)Au(s)< H2O(l)< CaCO3(s)
D)CaCO3(s)< Au(s)< H2O(l)
A)Au(s)< CaCO3(s)< H2O(l)
B)CaCO3(s)< H2O(l)< Au(s)
C)Au(s)< H2O(l)< CaCO3(s)
D)CaCO3(s)< Au(s)< H2O(l)

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
23
Which set below has the species listed in order of increasing standard entropy, S°?
A)NaHCO3(aq)< C2H5OH(l)< Cr(s)< N2(g)
B)Cr(s)< N2(g)< NaHCO3(aq)< C2H5OH(l)
C)Cr(s)< C2H5OH(l)< NaHCO3(aq)< N2(g)
D)Cr(s)< NaHCO3(aq)< C2H5OH(l)< N2(g)
E)N2(g)< NaHCO3(aq)< Cr(s)< C2H5OH(l)
A)NaHCO3(aq)< C2H5OH(l)< Cr(s)< N2(g)
B)Cr(s)< N2(g)< NaHCO3(aq)< C2H5OH(l)
C)Cr(s)< C2H5OH(l)< NaHCO3(aq)< N2(g)
D)Cr(s)< NaHCO3(aq)< C2H5OH(l)< N2(g)
E)N2(g)< NaHCO3(aq)< Cr(s)< C2H5OH(l)

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
24
Which set below has the species listed in order of increasing standard entropy, S°?
A)CaSO4(s)< C2H5OH(l)< Ar(g)
B)CH3CH2-O-H(l)< Ar(g)< CaSO4(s)
C)CaSO4(s)< Ar(g)< C2H5OH(l)
D)C2H5OH(l)< CaSO4(s)< Ar(g)
A)CaSO4(s)< C2H5OH(l)< Ar(g)
B)CH3CH2-O-H(l)< Ar(g)< CaSO4(s)
C)CaSO4(s)< Ar(g)< C2H5OH(l)
D)C2H5OH(l)< CaSO4(s)< Ar(g)

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
25
For a certain chemical reaction, H is < 0 and S is < 0. This means that
A)we conclude the reaction must be spontaneous regardless of temperature and becomes even more so at higher temperatures.
B)we conclude the reaction must be spontaneous regardless of temperature and becomes even more so at lower temperatures.
C)we conclude the reaction may or may not be spontaneous, but spontaneity is favored by low temperatures.
D)we conclude the reaction may or may not be spontaneous, but spontaneity is favored by high temperatures.
E)we cannot make any conclusion about spontaneity or even tendencies from the limited information presented.
A)we conclude the reaction must be spontaneous regardless of temperature and becomes even more so at higher temperatures.
B)we conclude the reaction must be spontaneous regardless of temperature and becomes even more so at lower temperatures.
C)we conclude the reaction may or may not be spontaneous, but spontaneity is favored by low temperatures.
D)we conclude the reaction may or may not be spontaneous, but spontaneity is favored by high temperatures.
E)we cannot make any conclusion about spontaneity or even tendencies from the limited information presented.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
26
For a certain chemical reaction, H is > 0 and S is < 0. This means that
A)we conclude the reaction must be nonspontaneous regardless of temperature.
B)we conclude the reaction must be spontaneous regardless of temperature and becomes even more so at lower temperatures.
C)we conclude the reaction may or may not be spontaneous, but spontaneity is favored by low temperatures.
D)we conclude the reaction may or may not be spontaneous, but spontaneity is favored by high temperatures.
E)we cannot make any conclusion about spontaneity or even tendencies from the limited information presented.
A)we conclude the reaction must be nonspontaneous regardless of temperature.
B)we conclude the reaction must be spontaneous regardless of temperature and becomes even more so at lower temperatures.
C)we conclude the reaction may or may not be spontaneous, but spontaneity is favored by low temperatures.
D)we conclude the reaction may or may not be spontaneous, but spontaneity is favored by high temperatures.
E)we cannot make any conclusion about spontaneity or even tendencies from the limited information presented.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
27
The requirement for a spontaneous chemical reaction is
A) G = 0.
B) H > 0.
C) S = 0.
D) E > 0.
E) G < 0.
A) G = 0.
B) H > 0.
C) S = 0.
D) E > 0.
E) G < 0.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
28
The normal melting point of benzoic acid is 122.4°C. Predict the signs of H, S, and ?G for the process in which liquid benzoic acid freezes at 120°C and 1 atm: C7H6O2(l).C7H6O2(s) 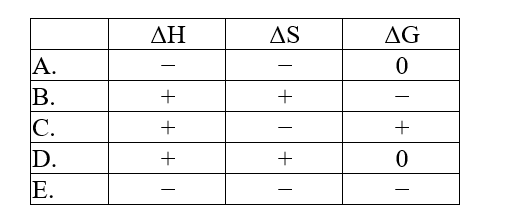
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
29
The normal melting point of carbon dioxide is -78°C. Predict the signs of H, S, and G for the process in which solid carbon dioxide sublimes at -50°C and 1 atm: CO2(s).CO2(g) 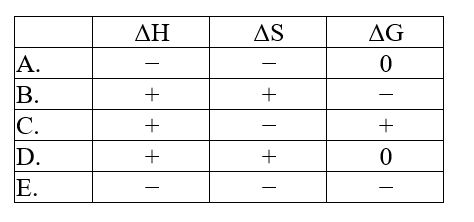
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
30
The normal melting point of naphthalene is 80.3°C. Predict the signs of H, S, and G for the process in which solid naphthalene melts at 82.5°C and 1 atm: C10H8(s). C10H8(l) 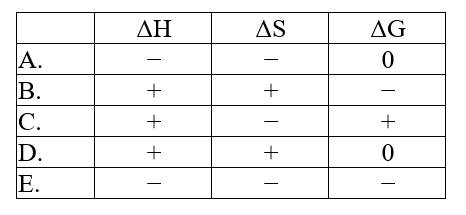
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
31
Which property associated with a chemical reaction is dependent on how the reaction is carried out, not on just its initial and final states?
A) S
B) H
C) E
D)w
E) G
A) S
B) H
C) E
D)w
E) G

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
32
A negative sign for G indicates that, at constant temperature and pressure,
A)the reaction is spontaneous.
B) S must be greater than zero.
C)the reaction must be exothermic.
D)the reaction must be fast.
E)the reaction must be endothermic.
A)the reaction is spontaneous.
B) S must be greater than zero.
C)the reaction must be exothermic.
D)the reaction must be fast.
E)the reaction must be endothermic.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
33
For the reaction 2NO(g)+ O2(g) 2NO2(g), H° = -113.1 kJ/mol and S° = -145.3 J/K mol.Which of these statements is true?
A)The reaction is spontaneous at all temperatures.
B)The reaction is only spontaneous at low temperatures.
C)The reaction is only spontaneous at high temperatures.
D)The reaction is at equilibrium at 25°C under standard conditions.
E) G° becomes more favorable as temperature increases.
A)The reaction is spontaneous at all temperatures.
B)The reaction is only spontaneous at low temperatures.
C)The reaction is only spontaneous at high temperatures.
D)The reaction is at equilibrium at 25°C under standard conditions.
E) G° becomes more favorable as temperature increases.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
34
According to the second law of thermodynamics, a spontaneous reaction must result in
A)no change in free energy for the reaction.
B)the enthalpy of the universe increasing.
C)the entropy of the universe decreasing.
D)the temperature of the surrounding increasing.
E)the entropy of the universe increasing.
A)no change in free energy for the reaction.
B)the enthalpy of the universe increasing.
C)the entropy of the universe decreasing.
D)the temperature of the surrounding increasing.
E)the entropy of the universe increasing.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
35
Using the standard entropy values:
H2(g), S° = +130.6 J mol-1 K-1
I2(s), S° = +116.12 J mol-1 K-1
HI(g), S° = +206.0 J mol-1 K-1
Calculate the standard entropy change, S°, for the reaction:H2(g)+ I2(s) 2 HI(g)
A)-40.8 J mol-1 K-1
B)+40.8 J mol-1 K-1
C)-165.3 J mol-1 K-1
D)+165.3 J mol-1 K-1
E)+206.0 J mol-1 K-1
H2(g), S° = +130.6 J mol-1 K-1
I2(s), S° = +116.12 J mol-1 K-1
HI(g), S° = +206.0 J mol-1 K-1
Calculate the standard entropy change, S°, for the reaction:H2(g)+ I2(s) 2 HI(g)
A)-40.8 J mol-1 K-1
B)+40.8 J mol-1 K-1
C)-165.3 J mol-1 K-1
D)+165.3 J mol-1 K-1
E)+206.0 J mol-1 K-1

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
36
Using the standard entropy values:
NO(g), S° = +210.6 J mol-1 K-1
O2(g), S° = +205.0 J mol-1 K-1
NO2(g), S° = +240.5 J mol-1 K-1
Calculate the standard entropy change, S°, for the reaction:NO(g)+ ½ O2(g) NO2(g)
A)-72.6 J mol-1 K-1
B)-175.1 J mol-1 K-1
C)+72.6 J mol-1 K-1
D)+175.1 J mol-1 K-1
E)-656.1 J mol-1 K-1
NO(g), S° = +210.6 J mol-1 K-1
O2(g), S° = +205.0 J mol-1 K-1
NO2(g), S° = +240.5 J mol-1 K-1
Calculate the standard entropy change, S°, for the reaction:NO(g)+ ½ O2(g) NO2(g)
A)-72.6 J mol-1 K-1
B)-175.1 J mol-1 K-1
C)+72.6 J mol-1 K-1
D)+175.1 J mol-1 K-1
E)-656.1 J mol-1 K-1

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
37
Using the standard entropy values:
C2H4(g), S° = +219.8 J mol-1 K-
H2(g), S° = +130.6 J mol-1 K-1
C2H6(g), S° = +229.5 J mol-1 K-1
Calculate the standard entropy change, S°, for the reaction:C2H4(g)+ H2(g) C2H6(g)
A)+120.9 J mol-1 K-1
B)-98.9 J mol-1 K-1
C)-120.9 J mol-1 K-1
D)+140.3 J mol-1 K-1
E)+579.9 J mol-1 K-1
C2H4(g), S° = +219.8 J mol-1 K-
H2(g), S° = +130.6 J mol-1 K-1
C2H6(g), S° = +229.5 J mol-1 K-1
Calculate the standard entropy change, S°, for the reaction:C2H4(g)+ H2(g) C2H6(g)
A)+120.9 J mol-1 K-1
B)-98.9 J mol-1 K-1
C)-120.9 J mol-1 K-1
D)+140.3 J mol-1 K-1
E)+579.9 J mol-1 K-1

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
38
Using the standard entropy values:
SO2(g), S° = +248.0 J mol-1 K-1
SO3(g), S° = +256.0 J mol-1 K-1
NO(g), S° = +210.6 J mol-1 K-1
NO2(g), S° = +240.5 J mol-1 K-1
Calculate the standard entropy change, S°, for the reaction:NO2(g)+ SO2(g) NO(g)+ SO3(g)
A)-6.2 J mol-1 K-1
B)+21.9 J mol-1 K-1
C)-21.9 J mol-1 K-1
D)-37.9 J mol-1 K-1
E)+52.9 J mol-1 K-1
SO2(g), S° = +248.0 J mol-1 K-1
SO3(g), S° = +256.0 J mol-1 K-1
NO(g), S° = +210.6 J mol-1 K-1
NO2(g), S° = +240.5 J mol-1 K-1
Calculate the standard entropy change, S°, for the reaction:NO2(g)+ SO2(g) NO(g)+ SO3(g)
A)-6.2 J mol-1 K-1
B)+21.9 J mol-1 K-1
C)-21.9 J mol-1 K-1
D)-37.9 J mol-1 K-1
E)+52.9 J mol-1 K-1

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
39
Using the standard free energies of formation:
BaCO3(s), G°f = -1139.0 kJ mol-1
BaSO4(s), G°f = -1353.0 kJ mol-1
CO2(g), G°f = -394.4 kJ mol-1
SO3(g), G°f = -370.0 kJ mol-1
Calculate the standard free energy change, G°, for the reaction:BaCO3(s)+ SO3(g) BaSO4(s)+ CO2(g)
A)+189.6 kJ mol-1
B)-238.4 kJ mol-1
C)+472.4 kJ mol-1
D)+238.4 kJ mol-1
E)-2516.4 kJ mol-1
BaCO3(s), G°f = -1139.0 kJ mol-1
BaSO4(s), G°f = -1353.0 kJ mol-1
CO2(g), G°f = -394.4 kJ mol-1
SO3(g), G°f = -370.0 kJ mol-1
Calculate the standard free energy change, G°, for the reaction:BaCO3(s)+ SO3(g) BaSO4(s)+ CO2(g)
A)+189.6 kJ mol-1
B)-238.4 kJ mol-1
C)+472.4 kJ mol-1
D)+238.4 kJ mol-1
E)-2516.4 kJ mol-1

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
40
Using the standard free energies of formation:
NO2(g), G°f = +51.84 kJ mol-1
NO(g), G°f = +86.69 kJ mol-1
SO2(g), G°f = -300.0 kJ mol-1
SO3(g), G°f = -370.0 kJ mol-1
Calculate the standard free energy change, G°, for the reaction:NO2(g)+ SO2(g) NO(g)+ SO3(g)
A)-35.15 kJ mol-1
B)-104.9 kJ mol-1
C)-429.2 kJ mol-1
D)-619.6 kJ mol-1
E)+35.15 kJ mol-1
NO2(g), G°f = +51.84 kJ mol-1
NO(g), G°f = +86.69 kJ mol-1
SO2(g), G°f = -300.0 kJ mol-1
SO3(g), G°f = -370.0 kJ mol-1
Calculate the standard free energy change, G°, for the reaction:NO2(g)+ SO2(g) NO(g)+ SO3(g)
A)-35.15 kJ mol-1
B)-104.9 kJ mol-1
C)-429.2 kJ mol-1
D)-619.6 kJ mol-1
E)+35.15 kJ mol-1

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
41
Given the data:
H2(g), H°f = 0 kJ mol-1, S° = +130.6 J mol-1 K-1
I2(s), H°f = 0 kJ mol-1, S° = +116.12 J mol-1 K-1
HI(g), H°f = +26 kJ mol-1, S° = +206 J mol-1 K-1
Calculate the standard free energy change, G°, for the reaction:H2(g)+ I2(s) 2 HI(g)
A)+2.7 kJ mol-1
B)-46.5 kJ mol-1
C)-2.7 kJ mol-
D)+128.2 kJ mol-1
E)-165.3 kJ mol-1
H2(g), H°f = 0 kJ mol-1, S° = +130.6 J mol-1 K-1
I2(s), H°f = 0 kJ mol-1, S° = +116.12 J mol-1 K-1
HI(g), H°f = +26 kJ mol-1, S° = +206 J mol-1 K-1
Calculate the standard free energy change, G°, for the reaction:H2(g)+ I2(s) 2 HI(g)
A)+2.7 kJ mol-1
B)-46.5 kJ mol-1
C)-2.7 kJ mol-
D)+128.2 kJ mol-1
E)-165.3 kJ mol-1

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
42
Given the data:
Ag2O(s), H°f = -31.1 kJ mol-1, S° = +121.3 J mol-1 K-1
O2(g), H°f = 0.00 kJ mol-1, S° = +205 J mol-1 K-1
Ag(s), H°f = 0.00 kJ mol-1, S° = +42.55 J mol-1 K-1
Calculate the standard free energy change G°, for the reaction:Ag2O(s) 2 Ag(s)+ ½ O2(g)
A)+11.3 kJ mol-1
B)-24.0 kJ mol-1
C)-38.2 kJ mol-1
D)-50.4 kJ mol-1
E)-1.3 kJ mol-1
Ag2O(s), H°f = -31.1 kJ mol-1, S° = +121.3 J mol-1 K-1
O2(g), H°f = 0.00 kJ mol-1, S° = +205 J mol-1 K-1
Ag(s), H°f = 0.00 kJ mol-1, S° = +42.55 J mol-1 K-1
Calculate the standard free energy change G°, for the reaction:Ag2O(s) 2 Ag(s)+ ½ O2(g)
A)+11.3 kJ mol-1
B)-24.0 kJ mol-1
C)-38.2 kJ mol-1
D)-50.4 kJ mol-1
E)-1.3 kJ mol-1

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
43
Given the data:
N2(g), H°f = 0.00 kJ mol-1, S° = +191.5 J mol-1 K-1
H2(g), H°f = 0.00 kJ mol-1, S° = +130.6 J mol-1 K-1
NH3(g), H°f = -46.0 kJ mol-1, S° = +192.5 J mol-1 K-1
Calculate the standard free energy change, G° for the reaction:N2(g)+ 3 H2(g) 2 NH3(g)
A)-7.4 kJ mol-1
B)-32.9 kJ mol-1
C)-84.6 kJ mol-1
D)+112.3 kJ mol-1
E)+32.9 kJ mol-1
N2(g), H°f = 0.00 kJ mol-1, S° = +191.5 J mol-1 K-1
H2(g), H°f = 0.00 kJ mol-1, S° = +130.6 J mol-1 K-1
NH3(g), H°f = -46.0 kJ mol-1, S° = +192.5 J mol-1 K-1
Calculate the standard free energy change, G° for the reaction:N2(g)+ 3 H2(g) 2 NH3(g)
A)-7.4 kJ mol-1
B)-32.9 kJ mol-1
C)-84.6 kJ mol-1
D)+112.3 kJ mol-1
E)+32.9 kJ mol-1

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
44
Given the data:
NH3(g), H°f = -46.0 kJ mol-1, S° = +192.5 J mol-1 K-1
NO(g), H°f = +90.4 kJ mol-1, S° = +210.6 J mol-1 K-1
H2O(l), H°f = -286 kJ mol-1, S° = +69.96 J mol-1 K-1
O2(g), H°f = 0.00 kJ mol-1, S° = +205 J mol-1 K-1
Calculate the standard free energy change, G°, for the reaction:2 NH3(g)+ 5/2 O2(g) 2 NO(g)+ 3 H2O(l)
A)-100.8 kJ mol-1
B)-206.7 kJ mol-1
C)-276.5 kJ mol-1
D)-505.8 kJ mol-1
E)+664.3 kJ mol-1
NH3(g), H°f = -46.0 kJ mol-1, S° = +192.5 J mol-1 K-1
NO(g), H°f = +90.4 kJ mol-1, S° = +210.6 J mol-1 K-1
H2O(l), H°f = -286 kJ mol-1, S° = +69.96 J mol-1 K-1
O2(g), H°f = 0.00 kJ mol-1, S° = +205 J mol-1 K-1
Calculate the standard free energy change, G°, for the reaction:2 NH3(g)+ 5/2 O2(g) 2 NO(g)+ 3 H2O(l)
A)-100.8 kJ mol-1
B)-206.7 kJ mol-1
C)-276.5 kJ mol-1
D)-505.8 kJ mol-1
E)+664.3 kJ mol-1

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
45
Given the data:
PbO(s), H°f = -217.3 kJ mol-1, S° = +68.7 J mol-1 K?1
PbO2(s), H°f = -277.0 kJ mol-1, S° = +68.6 J mol-1K-1
O2(g), H°f = 0.00 kJ mol-1, S° = +205 J mol-1 K-1
Calculate the standard free energy change, ?G°, for the reaction:PbO(s)+ ½ O2(g) PbO2(s)
A)+1.45 kJ mol-1
B)-29.1 kJ mol-1
C)-68.3 kJ mol-1
D)-90.3 kJ mol-1
E)+29.1 kJ mol-1
PbO(s), H°f = -217.3 kJ mol-1, S° = +68.7 J mol-1 K?1
PbO2(s), H°f = -277.0 kJ mol-1, S° = +68.6 J mol-1K-1
O2(g), H°f = 0.00 kJ mol-1, S° = +205 J mol-1 K-1
Calculate the standard free energy change, ?G°, for the reaction:PbO(s)+ ½ O2(g) PbO2(s)
A)+1.45 kJ mol-1
B)-29.1 kJ mol-1
C)-68.3 kJ mol-1
D)-90.3 kJ mol-1
E)+29.1 kJ mol-1

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
46
Using the data:
C2H4(g), H°f = +51.9 kJ mol-1, S° = 219.8 J mol-1 K-1
CO2(g), H°f = -394 kJ mol-1, S° = 213.6 J mol+1 K-1
H2O(l), H°f = -286.0 kJ mol-1, S° = 69.96 J mol-1 K-1
O2(g), H°f = 0.00 kJ mol-1, S° = 205 J mol-1 K-1
Calculate the maximum amount of work that can be obtained, at 25.0 °C, from the process:C2H4(g)+ 3 O2(g) 2 CO2(g)+ 2 H2O(l)
A)1332 kJ mol-1
B)1380 kJ mol-1
C)1451 kJ mol-1
D)1492 kJ mol-1
E)2422 kJ mol-1
C2H4(g), H°f = +51.9 kJ mol-1, S° = 219.8 J mol-1 K-1
CO2(g), H°f = -394 kJ mol-1, S° = 213.6 J mol+1 K-1
H2O(l), H°f = -286.0 kJ mol-1, S° = 69.96 J mol-1 K-1
O2(g), H°f = 0.00 kJ mol-1, S° = 205 J mol-1 K-1
Calculate the maximum amount of work that can be obtained, at 25.0 °C, from the process:C2H4(g)+ 3 O2(g) 2 CO2(g)+ 2 H2O(l)
A)1332 kJ mol-1
B)1380 kJ mol-1
C)1451 kJ mol-1
D)1492 kJ mol-1
E)2422 kJ mol-1

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following is the best example of a thermodynamically reversible process?
A)water dripping out of a leak in a pail
B)the conversion of ice to water at 0°C
C)the popping of a balloon
D)the dissolving of sugar into water
E)the cracking of a block of ice with a hammer
A)water dripping out of a leak in a pail
B)the conversion of ice to water at 0°C
C)the popping of a balloon
D)the dissolving of sugar into water
E)the cracking of a block of ice with a hammer

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is the best example of a thermodynamically reversible process?
A)a nail rusting in a deck
B)the dispersion of food coloring when added to a glass of water
C)the buring of a piece of paper
D)the conversion of water to ice in a sealed at 0°C
E)the conversion of dry ice to CO2 gas on a counter at room temperature
A)a nail rusting in a deck
B)the dispersion of food coloring when added to a glass of water
C)the buring of a piece of paper
D)the conversion of water to ice in a sealed at 0°C
E)the conversion of dry ice to CO2 gas on a counter at room temperature

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
49
In order to get the most efficient work out of a thermochemical process, what needs to be done?
A)The process must take place at high temperature.
B)The process must be endothermic.
C)The process must be exothermic.
D)The process must be done reversibly.
E)The process must cause an increase in entropy for the system.
A)The process must take place at high temperature.
B)The process must be endothermic.
C)The process must be exothermic.
D)The process must be done reversibly.
E)The process must cause an increase in entropy for the system.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
50
Using the data:
C2H6(g), H°f = -84.5 kJ mol-1, S° = +229.5 J mol-1 K-1
CO2(g), H°f = -394.0 kJ mol-1, S° = +213.6 J mol-1 K-1
H2O(l), H°f = -286.0 kJ mol-1, S° = +69.96 J mol-1 K-1
O2(g), H°f = 0.00 kJ mol-1, S° = +205 J mol-1 K-1
Calculate the maximum amount of work that can be obtained, at 25.0 °C, from the process:C2H6(g)+ 7 O2(g) 4 CO2(g)+ 6 H2O(l), per mole of C2H6
A)1426 kJ mol-1
B)1469 kJ mol-1
C)1654 kJ mol-1
D)2938 kJ mol-1
E)3029 kJ mol-1
C2H6(g), H°f = -84.5 kJ mol-1, S° = +229.5 J mol-1 K-1
CO2(g), H°f = -394.0 kJ mol-1, S° = +213.6 J mol-1 K-1
H2O(l), H°f = -286.0 kJ mol-1, S° = +69.96 J mol-1 K-1
O2(g), H°f = 0.00 kJ mol-1, S° = +205 J mol-1 K-1
Calculate the maximum amount of work that can be obtained, at 25.0 °C, from the process:C2H6(g)+ 7 O2(g) 4 CO2(g)+ 6 H2O(l), per mole of C2H6
A)1426 kJ mol-1
B)1469 kJ mol-1
C)1654 kJ mol-1
D)2938 kJ mol-1
E)3029 kJ mol-1

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
51
Assuming that, since the physical states do not change, the values of ?H and sS do not change as we raise the temperature in the reaction below. Using the following values at 298 K,
CdO(s), H°f = -258.2 kJ mol-1, S° = +54.8 J mol-1 K-1
CdSO4(s), H°f = -933.5 kJ mol-1, S° = +123 J mol-1 K?1
SO3(g), H°f = -396 kJ mol-1, S° = +256 J mol-1 K-1
Calculate a value for the free energy change, G°T for the reaction,CdSO4(s) CdO(s)+ SO3(g)at 750 K
A)+223.3 kJ mol-1
B)+138.5 kJ mol-1
C)-140.6 kJ mol-1
D)+420.5 kJ mol-1
E)-420.5 kJ mol-1
CdO(s), H°f = -258.2 kJ mol-1, S° = +54.8 J mol-1 K-1
CdSO4(s), H°f = -933.5 kJ mol-1, S° = +123 J mol-1 K?1
SO3(g), H°f = -396 kJ mol-1, S° = +256 J mol-1 K-1
Calculate a value for the free energy change, G°T for the reaction,CdSO4(s) CdO(s)+ SO3(g)at 750 K
A)+223.3 kJ mol-1
B)+138.5 kJ mol-1
C)-140.6 kJ mol-1
D)+420.5 kJ mol-1
E)-420.5 kJ mol-1

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
52
Assuming that, since the physical states do not change, the values of H and S do not change as we raise the temperature in the reaction below. Using the following values at 25 °C,
CaO(s), H°f = -635.5 kJ mol-1, S° = +40.0 J mol-1 K-1
CaCO3(s), H°f = -1207 kJ mol-1, S° = +92.9 J mol-1 K-1
CO2(g), H°f = -394 kJ mol-1, S° = +213.6 J mol-1 K-1
Calculate a value for the free energy change, G°T for the reaction,CaCO3(s) CaO(s)+ CO2(g)at 815 °C
A)+2.6 kJ mol-1
B)+46.5 kJ mol-1
C)-174.7 kJ mol-1
D)+308.5 kJ mol-1
E)-352.4 kJ mol-1
CaO(s), H°f = -635.5 kJ mol-1, S° = +40.0 J mol-1 K-1
CaCO3(s), H°f = -1207 kJ mol-1, S° = +92.9 J mol-1 K-1
CO2(g), H°f = -394 kJ mol-1, S° = +213.6 J mol-1 K-1
Calculate a value for the free energy change, G°T for the reaction,CaCO3(s) CaO(s)+ CO2(g)at 815 °C
A)+2.6 kJ mol-1
B)+46.5 kJ mol-1
C)-174.7 kJ mol-1
D)+308.5 kJ mol-1
E)-352.4 kJ mol-1

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
53
Naphthalene (C10H8)is a solid at room temperature. It sublimes by the following process. Using the data below determine the best estimate of the sublimation temperature for naphthalene (the temperature at which this reaction will become spontaneous under standard state conditions.
C10H8(s) C10H8(g)
H° = 72.1 kJ/mol
S° = 196.55 J/K mol
A)2.73 K
B)421 K
C)561 K
D)367 K
E)315 K
C10H8(s) C10H8(g)
H° = 72.1 kJ/mol
S° = 196.55 J/K mol
A)2.73 K
B)421 K
C)561 K
D)367 K
E)315 K

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
54
Assuming that, since the physical states do not change, the values of H and S do not change as we raise the temperature, and using,
N2(g), H°f = 0.00 kJ mol-1, S° = +191.5 J mol-1 K-1
H2(g), H°f = 0.00 kJ mol-1, S° = +130.6 J mol-1 K-1
NH3(g), H°f = -46.0 kJ mol-1, S° = +192.5 J mol-1 K-1
Calculate a value for the free energy change, G°T for the reaction below, at 500 °C N2(g)+ 3 H2(g) 2 NH3(g)
A)+7.2 kJ mol-1
B)+245.3 kJ mol-1
C)+99.1 kJ mol-1
D)+61.3 kJ mol-1
E)+153.2 kJ mol-1
N2(g), H°f = 0.00 kJ mol-1, S° = +191.5 J mol-1 K-1
H2(g), H°f = 0.00 kJ mol-1, S° = +130.6 J mol-1 K-1
NH3(g), H°f = -46.0 kJ mol-1, S° = +192.5 J mol-1 K-1
Calculate a value for the free energy change, G°T for the reaction below, at 500 °C N2(g)+ 3 H2(g) 2 NH3(g)
A)+7.2 kJ mol-1
B)+245.3 kJ mol-1
C)+99.1 kJ mol-1
D)+61.3 kJ mol-1
E)+153.2 kJ mol-1

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
55
Over a wide temperature range, the reaction, M2O3(s)+ C(s) M(s)+ CO2(g), is spontaneous at low temperatures but non-spontaneous at high temperatures. If we assume that, since the physical states do not change, the values of G°T and S°T are constant over this temperature range, we can then deduce that
A) H < 0 and S > 0.
B) H < 0 and S < 0.
C) H > 0 and S < 0.
D) H > 0 and S > 0.
E)The information is insufficient to make any judgment as to the signs of H and S.
A) H < 0 and S > 0.
B) H < 0 and S < 0.
C) H > 0 and S < 0.
D) H > 0 and S > 0.
E)The information is insufficient to make any judgment as to the signs of H and S.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
56
Given the data:
Ag2O(s), H°f = -31.1 kJ mol-1, S° = +121.3 J mol-1 K-1
Ag(s), H°f = 0.00 kJ mol-1, S° = +42.55 J mol-1 K-1
O2(g), H°f = 0.00 kJ mol-1, S° = +205.0 J mol-1 K-1
Calculate the temperature at which G°T = 0 for the reaction, Ag2O(s) 2 Ag(s)+ ½ O2(g). Assume that, since the physical states do not change, H° and S° are independent of temperature between -50.0 °C and 950.0 °C.
A)+196 °C
B)+246 °C
C)+423 °C
D)+610 °C
E)+818 °C
Ag2O(s), H°f = -31.1 kJ mol-1, S° = +121.3 J mol-1 K-1
Ag(s), H°f = 0.00 kJ mol-1, S° = +42.55 J mol-1 K-1
O2(g), H°f = 0.00 kJ mol-1, S° = +205.0 J mol-1 K-1
Calculate the temperature at which G°T = 0 for the reaction, Ag2O(s) 2 Ag(s)+ ½ O2(g). Assume that, since the physical states do not change, H° and S° are independent of temperature between -50.0 °C and 950.0 °C.
A)+196 °C
B)+246 °C
C)+423 °C
D)+610 °C
E)+818 °C

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
57
The thermochemical equation representing one process used for the synthesis of ammonia involves the equilibrium shown,N2(g)+ 3 H2(g)  2 NH3(g)or this reaction, ?H° = -92.2 kJ, G° = -33.4 kJ. All things considered, one concludes that
2 NH3(g)or this reaction, ?H° = -92.2 kJ, G° = -33.4 kJ. All things considered, one concludes that
A)the coefficients give us the mole ratios but not the volume ratios of the reacting species.
B)an increase in pressure favors an increase in the yield of ammonia.
C)carrying out the reaction at a higher temperature shifts the position of equilibrium to the right, thus favoring an increase in the yield of ammonia.
D)cooling the mixture to remove ammonia from the reaction mixture unfortunately also decreases the overall yield of ammonia.
E)the mixture does not need heating, in fact, cooling the mixture assists the rapid establishment of equilibrium.
 2 NH3(g)or this reaction, ?H° = -92.2 kJ, G° = -33.4 kJ. All things considered, one concludes that
2 NH3(g)or this reaction, ?H° = -92.2 kJ, G° = -33.4 kJ. All things considered, one concludes thatA)the coefficients give us the mole ratios but not the volume ratios of the reacting species.
B)an increase in pressure favors an increase in the yield of ammonia.
C)carrying out the reaction at a higher temperature shifts the position of equilibrium to the right, thus favoring an increase in the yield of ammonia.
D)cooling the mixture to remove ammonia from the reaction mixture unfortunately also decreases the overall yield of ammonia.
E)the mixture does not need heating, in fact, cooling the mixture assists the rapid establishment of equilibrium.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
58
Determine the equilibrium constant Kp at 25°C for the reaction:N2(g)+ 3H2(g)  2NH3(g)( G°f (NH3(g))= -16.6 kJ/mol)
2NH3(g)( G°f (NH3(g))= -16.6 kJ/mol)
A)1.52 × 10-6
B)2.60
C)8.28 × 10-2
D)13.4
E)6.60 × 105
 2NH3(g)( G°f (NH3(g))= -16.6 kJ/mol)
2NH3(g)( G°f (NH3(g))= -16.6 kJ/mol)A)1.52 × 10-6
B)2.60
C)8.28 × 10-2
D)13.4
E)6.60 × 105

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
59
Determine the equilibrium constant Kp at 25°C for the reaction:2NO(g)+ O2(g)  2NO2(g)( G°rxn = -69.7 kJ/mol)
2NO2(g)( G°rxn = -69.7 kJ/mol)
A)1.65 × 1012
B)8.28 × 10-2
C)2.60
D)13.4
E)6.07 × 10-13
 2NO2(g)( G°rxn = -69.7 kJ/mol)
2NO2(g)( G°rxn = -69.7 kJ/mol)A)1.65 × 1012
B)8.28 × 10-2
C)2.60
D)13.4
E)6.07 × 10-13

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
60
The equilibrium constant at 427°C for the reactionN2(g)+ 3H2(g)  2NH3(g)is Kp = 9.4 × 10-5. Calculate the value of G° for the reaction at this temperature.
2NH3(g)is Kp = 9.4 × 10-5. Calculate the value of G° for the reaction at this temperature.
A)56 kJ/mol
B)-56 kJ/mol
C)-33 kJ/mol
D)33 kJ/mol
E)1.3 J/mol
 2NH3(g)is Kp = 9.4 × 10-5. Calculate the value of G° for the reaction at this temperature.
2NH3(g)is Kp = 9.4 × 10-5. Calculate the value of G° for the reaction at this temperature.A)56 kJ/mol
B)-56 kJ/mol
C)-33 kJ/mol
D)33 kJ/mol
E)1.3 J/mol

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
61
The equilibrium constant at 25°C for the reaction 2NO(g)+ O2(g)  2NO2(g)is Kp = 1.65 × 1012. Calculate the value of G° for the reaction at this temperature.
2NO2(g)is Kp = 1.65 × 1012. Calculate the value of G° for the reaction at this temperature.
A)-4.09 kJ/mol
B)-5.85 kJ/mol
C)+5.85 kJ/mol
D)-69.7 kJ/mol
E)1.65 kJ/mol
 2NO2(g)is Kp = 1.65 × 1012. Calculate the value of G° for the reaction at this temperature.
2NO2(g)is Kp = 1.65 × 1012. Calculate the value of G° for the reaction at this temperature.A)-4.09 kJ/mol
B)-5.85 kJ/mol
C)+5.85 kJ/mol
D)-69.7 kJ/mol
E)1.65 kJ/mol

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
62
Using these bond energies, ?H°: 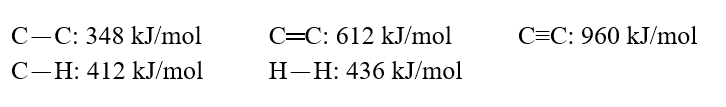 Calculate the value of H° of reaction for H2C.H2(g)+ H2(g) CH3-CH3(g)
Calculate the value of H° of reaction for H2C.H2(g)+ H2(g) CH3-CH3(g)
A)-560 kJ/mol
B)-124 kJ/mol
C)-388 kJ/mol
D)+224 kJ/mol
E)-212 kJ/mol
 Calculate the value of H° of reaction for H2C.H2(g)+ H2(g) CH3-CH3(g)
Calculate the value of H° of reaction for H2C.H2(g)+ H2(g) CH3-CH3(g)A)-560 kJ/mol
B)-124 kJ/mol
C)-388 kJ/mol
D)+224 kJ/mol
E)-212 kJ/mol

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
63
Using these bond energies, ?H°: 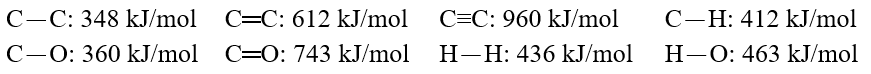 Calculate the value of H° of reaction for O.C.O(g)+ 3 H2(g). CH3-O-H(g)+ H-O-H(g)
Calculate the value of H° of reaction for O.C.O(g)+ 3 H2(g). CH3-O-H(g)+ H-O-H(g)
A)-191 kJ/mol
B)+272 kJ/mol
C)-272 kJ/mol
D)-5779 kJ/mol
E)+5779 kJ/mol
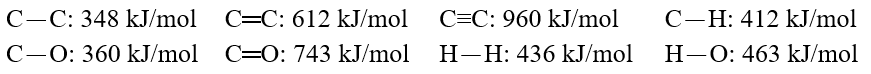 Calculate the value of H° of reaction for O.C.O(g)+ 3 H2(g). CH3-O-H(g)+ H-O-H(g)
Calculate the value of H° of reaction for O.C.O(g)+ 3 H2(g). CH3-O-H(g)+ H-O-H(g)A)-191 kJ/mol
B)+272 kJ/mol
C)-272 kJ/mol
D)-5779 kJ/mol
E)+5779 kJ/mol

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
64
Using these bond energies, H°: 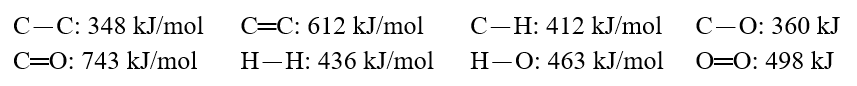 Calculate the value of H° of reaction for H2C.CH2(g)+ H-O-H(g) CH3-CH2-O-H(g)
Calculate the value of H° of reaction for H2C.CH2(g)+ H-O-H(g) CH3-CH2-O-H(g)
A)-13 kJ/mol
B)-45 kJ/mol
C)-124 kJ/mol
D)+224 kJ/mol
E)-508 kJ/mol
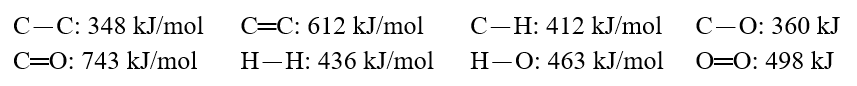 Calculate the value of H° of reaction for H2C.CH2(g)+ H-O-H(g) CH3-CH2-O-H(g)
Calculate the value of H° of reaction for H2C.CH2(g)+ H-O-H(g) CH3-CH2-O-H(g)A)-13 kJ/mol
B)-45 kJ/mol
C)-124 kJ/mol
D)+224 kJ/mol
E)-508 kJ/mol

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
65
According to the first law of thermodynamics the internal energy of an isolated system must ________.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
66
According to the first law of thermodynamics if the energy of a system decreases ________ amount of energy must be transferred to the surroundings.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
67
The ________ phase of a substance will always have more entropy then the liquid phase.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
68
The reaction N2(g)+ 3H2(g)→ 2NH3(g)would result in a(n)________ in entropy of the system.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
69
Consider the following chemical reaction: Cr3+(aq)+ 6H2O(l)→ [Cr(H2O)6]3+(aq)Would this reaction have a positive or negative entropy of formation?

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following processes are accompanied by an increase in entropy. One or more of the following answers may be selected.
1. 2SO2(g)+ O2(g)→ 2SO3(g)
2. H2O(l)→ H2O(s)
3. Br2(l)→ Br2(g)
4. H2O2(l)→ H2O(l)+ (1/2)O2(g)
1. 2SO2(g)+ O2(g)→ 2SO3(g)
2. H2O(l)→ H2O(s)
3. Br2(l)→ Br2(g)
4. H2O2(l)→ H2O(l)+ (1/2)O2(g)

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
71
According to the second law of thermodynamics, the entropy of the universe is always ________.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
72
For the reaction H2(g)+ S(s)→ H2S(g), ΔH° = -20.2 kJ/mol and ΔS° = +43.1 J/K·mol, at which temperatures would the reaction be spontaneous?

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
73
For the reaction 2NO(g)+ O2(g)→ 2NO2(g), ΔH° = −113.1 kJ/mol and ΔS° = -145.3 J/K·mol. Under which temperature conditions would the reaction be spontaneous?

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
74
Consider a reaction that is both exothermic and increases entropy. How will temperature affect the spontaneity of the process?

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
75
Consider a reaction that is both endothermic and increases entropy. How will temperature affect the spontaneity of the process?

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
76
Consider a reaction that is both exothermic and decreases order. How will temperature affect the spontaneity of the process?

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
77
Consider a reaction that is both endothermic and increasingly ordered. How will temperature affect the spontaneity of the process?

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
78
Describe, using the free energy relationship ΔG = ΔH - TΔS, the process of a hot object coming into thermal equilibrium with a cold object.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
79
HI has a normal boiling point of -35.4°C, and its ΔHvap is 21.16 kJ/mol. Calculate the molar entropy of vaporization (ΔSvap)for HI.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
80
The entropy of perfectly ordered pure crystalline substance at zero Kelvin is ________.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck


