Deck 18: Solubility and Simultaneous Equilibria
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/120
Play
Full screen (f)
Deck 18: Solubility and Simultaneous Equilibria
1
Which of the following is the expression for the solubility product of Ba3(AsO4)2?
A)Ksp = [Ba2+]3[AsO43]2
B)Ksp = [3 × Ba2+]3[2AsO43]2
C)Ksp = 3[Ba2+] 2[AsO43]
D)Ksp = 3[Ba2+]3 + 2[AsO43]2
E)Ksp = [Ba3+]3[AsO42]2
A)Ksp = [Ba2+]3[AsO43]2
B)Ksp = [3 × Ba2+]3[2AsO43]2
C)Ksp = 3[Ba2+] 2[AsO43]
D)Ksp = 3[Ba2+]3 + 2[AsO43]2
E)Ksp = [Ba3+]3[AsO42]2
Ksp = [Ba2+]3[AsO43]2
2
Which of the following is the expression for the solubility product of Ca3(PO4)2?
A)Ksp = [Ca2+]3 [PO43]2
B)Ksp = [3 × Ca2+]3[2PO43]2
C)Ksp = 3[Ca2+]2[PO43]
D)Ksp = 3[Ca2+]3 + 2[PO43]2
E)Ksp = [Ca2+]3[PO43]2
A)Ksp = [Ca2+]3 [PO43]2
B)Ksp = [3 × Ca2+]3[2PO43]2
C)Ksp = 3[Ca2+]2[PO43]
D)Ksp = 3[Ca2+]3 + 2[PO43]2
E)Ksp = [Ca2+]3[PO43]2
Ksp = [Ca2+]3 [PO43]2
3
Which of the following is the expression for the solubility product of Fe2(CrO4)3?
A)Ksp = [Fe2+]3[CrO43]2
B)Ksp = [2 × Fe2+]3[3CrO43]2
C)Ksp = 3[Fe2+] 2[CrO43]
D)Ksp = 2[Fe2+]3 + 3[CrO43]2
E)Ksp = [Fe3+]2[CrO42]3
A)Ksp = [Fe2+]3[CrO43]2
B)Ksp = [2 × Fe2+]3[3CrO43]2
C)Ksp = 3[Fe2+] 2[CrO43]
D)Ksp = 2[Fe2+]3 + 3[CrO43]2
E)Ksp = [Fe3+]2[CrO42]3
Ksp = [Fe3+]2[CrO42]3
4
Which of the following is the expression for the solubility product of copper(II)hydroxide?
A)Ksp = [Cu2+][2 OH]
B)Ksp = [Cu2+] 2[OH]2
C)Ksp = [Cu2+]2[OH]
D)Ksp = [Cu2+][OH]2
E)Ksp = [Cu2+] ½[OH]2
A)Ksp = [Cu2+][2 OH]
B)Ksp = [Cu2+] 2[OH]2
C)Ksp = [Cu2+]2[OH]
D)Ksp = [Cu2+][OH]2
E)Ksp = [Cu2+] ½[OH]2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is the expression for the solubility product of calcium fluoride, CaF2?
A)Ksp = [Ca2+][2 × F]
B)Ksp = [Ca2+] 2[F]2
C)Ksp = [Ca2+]2[F]
D)Ksp = [Ca2+][F]2
E)Ksp = [Ca2+] ½[F]2
A)Ksp = [Ca2+][2 × F]
B)Ksp = [Ca2+] 2[F]2
C)Ksp = [Ca2+]2[F]
D)Ksp = [Ca2+][F]2
E)Ksp = [Ca2+] ½[F]2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is the expression for the solubility product of silver oxalate (Ag2C2O4)?
A)Ksp = [Ag22+][C2O42]
B)Ksp = [Ag+][C2O42]2
C)Ksp = 2[Ag+][C2O42]
D)Ksp = [Ag+]2[C2O42]
E)Ksp = 2[Ag+]2[C2O42]
A)Ksp = [Ag22+][C2O42]
B)Ksp = [Ag+][C2O42]2
C)Ksp = 2[Ag+][C2O42]
D)Ksp = [Ag+]2[C2O42]
E)Ksp = 2[Ag+]2[C2O42]

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
7
The solubility of magnesium carbonate, MgCO3, in pure water is 2.6 × 10-4 moles per liter. Calculate the value of Ksp for magnesium carbonate from this data.
A)5.2 × 10-4
B)6.8 × 10-8
C)1.4 × 10-7
D)2.7 × 10-7
E)4.6 × 10-15
A)5.2 × 10-4
B)6.8 × 10-8
C)1.4 × 10-7
D)2.7 × 10-7
E)4.6 × 10-15

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
8
The solubility of calcium oxalate, CaC2O4, in pure water is 4.8 × 10-5 moles per liter. Calculate the value of Ksp for calcium oxalate from this data.
A)2.4 × 10-5
B)9.6 × 10-5
C)4.6 × 10-9
D)2.3 × 10-9
E)5.3 × 10-18
A)2.4 × 10-5
B)9.6 × 10-5
C)4.6 × 10-9
D)2.3 × 10-9
E)5.3 × 10-18

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
9
The solubility of scandium(III)fluoride, ScF3, in pure water is 2.0 × 10-5 moles per liter. Calculate the value of Ksp for scandium(III)fluoride from this data.
A)1.3 × 10-17
B)1.4 × 10-18
C)4.3 × 10-18
D)1.6 × 10-19
E)4.8 × 10-19
A)1.3 × 10-17
B)1.4 × 10-18
C)4.3 × 10-18
D)1.6 × 10-19
E)4.8 × 10-19

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
10
The solubility of lead(II)fluoride, PbF2, in pure water is 2.1 × 10-3 moles per liter. Calculate the value of Ksp for lead(II)fluoride from this data.
A)1.3 × 10-7
B)1.9 × 10-8
C)3.7 × 10-8
D)1.6 × 10-9
E)9.3 × 10-9
A)1.3 × 10-7
B)1.9 × 10-8
C)3.7 × 10-8
D)1.6 × 10-9
E)9.3 × 10-9

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
11
The solubility of calcium fluoride, CaF2, in pure water is 2.15 × 10-4 moles per liter. Calculate the value of Ksp for calcium fluoride from this data.
A)1.85 × 10-7
B)9.28 × 10-8
C)1.99 × 10-11
D)3.98 × 10-11
E)9.94 × 10-12
A)1.85 × 10-7
B)9.28 × 10-8
C)1.99 × 10-11
D)3.98 × 10-11
E)9.94 × 10-12

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
12
The solubility of copper(II)arsenate, Cu3(AsO4)2, in pure water is 3.7 × 10-8 moles per liter. Calculate the value of Ksp for copper(II)arsenate from this data.
A)6.9 × 10-38
B)4.2 × 10-37
C)2.5 × 10-36
D)3.7 × 10-36
E)7.5 × 10-36
A)6.9 × 10-38
B)4.2 × 10-37
C)2.5 × 10-36
D)3.7 × 10-36
E)7.5 × 10-36

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
13
The solubility of zinc(II)phosphate, Zn3(PO4)2, in pure water is 1.5 × 10-7 moles per liter. Calculate the value of Ksp for zinc(II)phosphate from this data.
A)2.3 × 10-14
B)5.1 × 10-28
C)2.7 × 10-33
D)8.2 × 10-33
E)7.6 × 10-35
A)2.3 × 10-14
B)5.1 × 10-28
C)2.7 × 10-33
D)8.2 × 10-33
E)7.6 × 10-35

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
14
The solubility of silver oxalate, Ag2C2O4, in pure water is 2.06 × 10-4 moles per liter. Calculate the value of Ksp for silver oxalate from this data.
A)4.24 × 10-8
B)8.49 × 10-8
C)1.75 × 10-11
D)3.50 × 10-11
E)8.74 × 10-12
A)4.24 × 10-8
B)8.49 × 10-8
C)1.75 × 10-11
D)3.50 × 10-11
E)8.74 × 10-12

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
15
The solubility of silver carbonate, Ag2CO3, in pure water is 1.27 × 10-4 moles per liter. Calculate the value of Ksp for silver carbonate from this data.
A)1.64 × 10-11
B)3.28 × 10-11
C)2.04 × 10-12
D)4.10 × 10-12
E)8.19 × 10-12
A)1.64 × 10-11
B)3.28 × 10-11
C)2.04 × 10-12
D)4.10 × 10-12
E)8.19 × 10-12

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
16
Which one of the following salts has the highest solubility in water, expressed in moles per liter?
A)PbF2, Ksp = 3.6 × 10-8
B)Ag2CrO4, Ksp = 1.2 × 10-12
C)CaF2, Ksp = 3.9 × 10-11
D)BaF2, Ksp = 1.7 × 10-6
E)PbI2, Ksp = 7.9 × 10-9
A)PbF2, Ksp = 3.6 × 10-8
B)Ag2CrO4, Ksp = 1.2 × 10-12
C)CaF2, Ksp = 3.9 × 10-11
D)BaF2, Ksp = 1.7 × 10-6
E)PbI2, Ksp = 7.9 × 10-9

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
17
Which one of the compounds below has the highest molar solubility in water?
A)SrF2, Ksp = 2.8 × 10-9
B)Sr(IO3)2, Ksp = 3.3 × 10-7
C)Fe(OH)2, Ksp = 8.0 × 10-16
D)PbCl2, Ksp = 1.6 × 10-5
E)PbBr2, Ksp = 3.9 × 10-5
A)SrF2, Ksp = 2.8 × 10-9
B)Sr(IO3)2, Ksp = 3.3 × 10-7
C)Fe(OH)2, Ksp = 8.0 × 10-16
D)PbCl2, Ksp = 1.6 × 10-5
E)PbBr2, Ksp = 3.9 × 10-5

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
18
Which one of the compounds below has the highest solubility in water, expressed in moles per liter?
A)SrF2, Ksp = 2.8 × 10-9
B)Sr(IO3)2, Ksp = 3.3 × 10-7
C)MgF2, Ksp = 6.5 × 10-9
D)PbCl2, Ksp = 1.6 × 10-5
E)BaF2, Ksp = 1.7 × 10-6
A)SrF2, Ksp = 2.8 × 10-9
B)Sr(IO3)2, Ksp = 3.3 × 10-7
C)MgF2, Ksp = 6.5 × 10-9
D)PbCl2, Ksp = 1.6 × 10-5
E)BaF2, Ksp = 1.7 × 10-6

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
19
Which one of the compounds below has the lowest solubility in water, expressed in moles per liter?
A)SrF2, Ksp = 2.8 × 10-9
B)Sr(IO3)2, Ksp = 3.3 × 10-7
C)MgF2, Ksp = 6.5 × 10-9
D)PbCl2, Ksp = 1.6 × 10-5
E)PbI2, Ksp = 7.9 × 10-9
A)SrF2, Ksp = 2.8 × 10-9
B)Sr(IO3)2, Ksp = 3.3 × 10-7
C)MgF2, Ksp = 6.5 × 10-9
D)PbCl2, Ksp = 1.6 × 10-5
E)PbI2, Ksp = 7.9 × 10-9

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
20
The solubility of silver sulfate (Ag2SO4), can be expressed in terms of the resulting ion concentrations. Which relationship is correct?
A)solubility = 2[Ag+]
B)solubility = [Ag+]
C)solubility = [2Ag+]
D)solubility = 2[SO42]
E)solubility = [SO42]
A)solubility = 2[Ag+]
B)solubility = [Ag+]
C)solubility = [2Ag+]
D)solubility = 2[SO42]
E)solubility = [SO42]

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
21
The solubility of strontium fluoride, SrF2, can be expressed in terms of the resulting ion concentrations. Which relationship is correct?
A)solubility = 2[Sr2+]
B)solubility = [Sr2+]
C)solubility = [2 Sr2+]
D)solubility = 2[F]
E)solubility = [F]
A)solubility = 2[Sr2+]
B)solubility = [Sr2+]
C)solubility = [2 Sr2+]
D)solubility = 2[F]
E)solubility = [F]

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
22
The solubility of copper(II)iodate, Cu(IO3)2, can be expressed in terms of the resulting ion concentrations. Which relationship is correct?
A)solubility = 2[Cu2+]
B)solubility = [Cu2+]
C)solubility = [2 Cu2+]
D)solubility = 2[IO3]
E)solubility = [IO3]2
A)solubility = 2[Cu2+]
B)solubility = [Cu2+]
C)solubility = [2 Cu2+]
D)solubility = 2[IO3]
E)solubility = [IO3]2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
23
The solubility of silver phosphate, Ag3PO4, can be expressed in terms of the resulting ion concentrations. Which relationship is correct?
A)solubility = 3[Ag+]
B)solubility = [Ag+]
C)solubility = [Ag+]3
D)solubility = [PO43-]
E)solubility = [PO43-]3
A)solubility = 3[Ag+]
B)solubility = [Ag+]
C)solubility = [Ag+]3
D)solubility = [PO43-]
E)solubility = [PO43-]3

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
24
The solubility product for Ag3PO4 is: Ksp = 2.8 × 10-18. What is the solubility of Ag3PO4 in water?
A)1.8 × 10-5 M
B)2.5 × 10-5 M
C)1.9 × 10-86 M
D)3.1 × 10-5 M
E)4.1 × 10-5 M
A)1.8 × 10-5 M
B)2.5 × 10-5 M
C)1.9 × 10-86 M
D)3.1 × 10-5 M
E)4.1 × 10-5 M

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
25
The solubility product for Ag3PO4 is: Ksp = 2.8 × 10-18. What is the solubility of Ag3PO4 in water, in grams per liter?
A)1.2 × 10-15 g/L
B)7.5 × 10-3 g/L
C)2.0 × 10-4 g/L
D)9.9 × 10-3 g/L
E)1.2 × 10-3 g/L
A)1.2 × 10-15 g/L
B)7.5 × 10-3 g/L
C)2.0 × 10-4 g/L
D)9.9 × 10-3 g/L
E)1.2 × 10-3 g/L

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
26
The solubility product for PbBr2 is: Ksp = 6.6 × 10-6. Which of the following represents the solubility of PbBr2?
A)4.7 × 10-1 g/L
B)2.4 × 10-3 g/L
C)6.0 × 10-4 g/L
D)4.3 g/L
E)6.1 × 10-4 g/L
A)4.7 × 10-1 g/L
B)2.4 × 10-3 g/L
C)6.0 × 10-4 g/L
D)4.3 g/L
E)6.1 × 10-4 g/L

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
27
The solubility product for BaSO4 is 1.1 × 10-10. Calculate the solubility of BaSO4 in pure water.
A)5.5 × 10-11 mol L-1
B)1.0 × 10-5 mol L-1
C)2.1 × 10-5 mol L-1
D)1.1 × 10-10 mol L-1
E)2.2 × 10-10 mol L-1
A)5.5 × 10-11 mol L-1
B)1.0 × 10-5 mol L-1
C)2.1 × 10-5 mol L-1
D)1.1 × 10-10 mol L-1
E)2.2 × 10-10 mol L-1

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
28
The solubility product for PbCl2 is 1.7 × 10-5. What is the solubility of PbCl2 in pure water?
A)2.4 × 10-4 mol L-1
B)6.2 × 10-2 mol L-1
C)7.7 × 10-3 mol L-1
D)1.6 × 10-2 mol L-1
E)6.0 × 10-5 mol L-1
A)2.4 × 10-4 mol L-1
B)6.2 × 10-2 mol L-1
C)7.7 × 10-3 mol L-1
D)1.6 × 10-2 mol L-1
E)6.0 × 10-5 mol L-1

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
29
Calculate the concentration of chloride ions in a saturated solution of lead(II)chloride. The Ksp = 1.7 × 10-5.
A)2.4 × 10-4 M
B)4.8 × 10-4 M
C)3.9 × 10-2 M
D)1.2 × 10-1 M
E)3.2 × 10-2 M
A)2.4 × 10-4 M
B)4.8 × 10-4 M
C)3.9 × 10-2 M
D)1.2 × 10-1 M
E)3.2 × 10-2 M

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
30
Calculate the concentration of bromide ions in a saturated solution of lead(II)bromide. The Ksp = 6.6 × 10-6.
A)1.2 × 10-2 M
B)2.2 × 10-2 M
C)2.0 × 10-2 M
D)1.2 × 10-1 M
E)2.1 × 10-3 M
A)1.2 × 10-2 M
B)2.2 × 10-2 M
C)2.0 × 10-2 M
D)1.2 × 10-1 M
E)2.1 × 10-3 M

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
31
Calculate the concentration of iodate ions in a saturated solution of lead(II)iodate, Pb(IO3)2. The Ksp = 2.6 × 10-13.
A)3.2 × 10-5 M
B)4.0 × 10-5 M
C)6.4 × 10-5 M
D)8.0 × 10-5 M
E)5.1 × 10-7 M
A)3.2 × 10-5 M
B)4.0 × 10-5 M
C)6.4 × 10-5 M
D)8.0 × 10-5 M
E)5.1 × 10-7 M

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
32
Calculate the concentration of iodate ions in a saturated solution of barium iodate, Ba(IO3)2. The Ksp = 1.5 × 10-9.
A)1.4 × 10-3 M
B)2.3 × 10-3 M
C)7.2 × 10-4 M
D)3.9 × 10-5 M
E)7.7 × 10-5 M
A)1.4 × 10-3 M
B)2.3 × 10-3 M
C)7.2 × 10-4 M
D)3.9 × 10-5 M
E)7.7 × 10-5 M

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
33
The solubility product of barium fluoride (BaF2)is 1.7 × 10-6. Calculate the concentration of fluoride ions in a saturated solution of barium fluoride.
A)7.6 × 10-3 M
B)1.5 × 10-2 M
C)3.4 × 10-5 M
D)1.7 × 10-6 M
E)3.4 × 10-6 M
A)7.6 × 10-3 M
B)1.5 × 10-2 M
C)3.4 × 10-5 M
D)1.7 × 10-6 M
E)3.4 × 10-6 M

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
34
The solubility product for Mg3(PO4)2 is 6.3 × 10-26. What is the solubility of Mg3(PO4)2 in pure water, in grams per liter?
A)1.7 × 10-23 g L-1
B)3.4 × 10-7 g L-1
C)9.4 × 10-4 g L-1
D)1.2 × 10-3 g L-1
E)2.4 × 10-3 g L-1
A)1.7 × 10-23 g L-1
B)3.4 × 10-7 g L-1
C)9.4 × 10-4 g L-1
D)1.2 × 10-3 g L-1
E)2.4 × 10-3 g L-1

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
35
The solubility of lead iodide is 578 mg L-1 at 25 °C. What is the Ksp for PbI2?
A)7.9 × 10-9
B)1.6 × 10-6
C)1.1 × 10-11
D)2.7 × 10-12
E)6.3 × 10-6
A)7.9 × 10-9
B)1.6 × 10-6
C)1.1 × 10-11
D)2.7 × 10-12
E)6.3 × 10-6

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
36
The solubility of barium carbonate is 14.8 mg L-1 at 30 °C. Calculate the Ksp value for BaCO3.
A)7.5 × 10-5
B)1.5 × 10-4
C)5.6 × 10-9
D)7.5 × 10-6
E)1.5 × 10-3
A)7.5 × 10-5
B)1.5 × 10-4
C)5.6 × 10-9
D)7.5 × 10-6
E)1.5 × 10-3

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
37
How many grams of lead(II)chloride would dissolve in 100 mL of water? The Ksp for lead(II)chloride is 2.4 × 10-4 and the molar mass is 278.1 g/mol.
A)1.1 g
B)0.039 g
C)10.9 g
D)4.3 g
E)1.4 × 10-4 g
A)1.1 g
B)0.039 g
C)10.9 g
D)4.3 g
E)1.4 × 10-4 g

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
38
What is the solubility, in moles per liter, of Fe(OH)2 (Ksp = 7.9 × 10-16), in 0.0500 molar NaOH solution?Hint: Be sure to account for the presence of a common ion. You should also be able to simplify the math because the solubility is very low.
A)3.16 × 10-13
B)3.13 × 10-16
C)1.58 × 10-14
D)1.14 × 10-14
E)3.16 × 10-16
A)3.16 × 10-13
B)3.13 × 10-16
C)1.58 × 10-14
D)1.14 × 10-14
E)3.16 × 10-16

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
39
What is the solubility, in moles per liter, of PbSO4 (Ksp = 6.3 × 10-7)in 0.0230 molar MgSO4 solution?Hint: Be sure to account for the presence of a common ion. You should also be able to simplify the math, because the solubility is very low.
A)2.14 × 10-5
B)7.24 × 10-5
C)2.74 × 10-5
D)4.72 × 10-5
E)4.27 × 10-5
A)2.14 × 10-5
B)7.24 × 10-5
C)2.74 × 10-5
D)4.72 × 10-5
E)4.27 × 10-5

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
40
What is the solubility, in moles per liter, of AgCl (Ksp = 1.8 × 10-10), in 0.0300 M CaCl2 solution?Hint: Be sure to account for the presence of a common ion and carefully organize. You should also be able to simplify the math because the solubility is very low.
A)9.0 × 10-3
B)9.0 × 10-6
C)6.0 × 10-9
D)3.0 × 10-9
E)6.0 × 10-3
A)9.0 × 10-3
B)9.0 × 10-6
C)6.0 × 10-9
D)3.0 × 10-9
E)6.0 × 10-3

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
41
What is the solubility, in moles per liter, of BaSO4 (Ksp = 1.1 × 10-10), in 0.0100 M Na2SO4 solution?Hint: Be sure to account for the presence of a common ion. You should also be able to simplify the math because the solubility is very low.
A)1.1 × 10-8
B)1.1 × 10-6
C)1.1 × 10-7
D)1.1 × 10-5
E)1.1 × 10-4
A)1.1 × 10-8
B)1.1 × 10-6
C)1.1 × 10-7
D)1.1 × 10-5
E)1.1 × 10-4

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
42
What is the solubility, in moles per liter, of MgCO3 (Ksp = 3.5 × 10-8), in 0.0200 M Na2CO3 solution?Hint: Be sure to account for the presence of a common ion. You should also be able to simplify the math because the solubility is very low.
A)4.4 × 10-6
B)8.1 × 10-4
C)4.4 × 10-3
D)8.8 × 10-5
E)1.8 × 10-6
A)4.4 × 10-6
B)8.1 × 10-4
C)4.4 × 10-3
D)8.8 × 10-5
E)1.8 × 10-6

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
43
What is the solubility, in moles per liter, of Ag2CO3 (Ksp = 8.1 × 10-12), in 0.0300 M Na2CO3 solution?Hint: Be sure to account for the presence of a common ion. You should also be able to simplify the math because the solubility is very low.
A)8.2 × 10-6
B)9.0 × 10-9
C)3.0 × 10-7
D)4.0 × 10-10
E)2.7 × 10-9
A)8.2 × 10-6
B)9.0 × 10-9
C)3.0 × 10-7
D)4.0 × 10-10
E)2.7 × 10-9

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
44
What is the solubility, in moles per liter, of AgCl (Ksp = 1.8 × 10-10), in 0.0100 molar aqueous potassium chloride solution?Hint: Be sure to account for the presence of a common ion. You should also be able to simplify the math because the solubility is very low.
A)7.5 × 10-5 mol L-1
B)1.8 × 10-8 mol L-1
C)1.3 × 10-6 mol L-1
D)3.6 × 10-8 mol L-1
E)1.5 × 10-7 mol L-1
A)7.5 × 10-5 mol L-1
B)1.8 × 10-8 mol L-1
C)1.3 × 10-6 mol L-1
D)3.6 × 10-8 mol L-1
E)1.5 × 10-7 mol L-1

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
45
The solubility of barium sulfate varies with the composition of the solvent in which it is dissolved. In which solvent mixture would BaSO4 have the lowest solubility? Assume all solutions are at 25 °C.
A)pure water
B)0.10 M Na2SO4(aq)
C)1.0 M (NH4)2SO4(aq)
D)0.5 M Ba(NO3)2(aq)
E)1.0 M HCl(aq)
A)pure water
B)0.10 M Na2SO4(aq)
C)1.0 M (NH4)2SO4(aq)
D)0.5 M Ba(NO3)2(aq)
E)1.0 M HCl(aq)

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
46
The solubility of PbI2 (Ksp = 9.8 × 10-9)varies with the composition of the solvent in which it was dissolved. In which solvent mixture would PbI2 have the lowest solubility at identical temperatures?
A)pure water
B)1.0 M Pb(NO3)2(aq)
C)1.5 M KI(aq)
D)0.8 M MgI2(aq)
E)1.0 M HCl(aq)
A)pure water
B)1.0 M Pb(NO3)2(aq)
C)1.5 M KI(aq)
D)0.8 M MgI2(aq)
E)1.0 M HCl(aq)

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
47
What is the maximum concentration of Mg2+ ion that can exist in a 0.10 M NaF(aq)solution, without precipitating any magnesium fluoride? The Ksp of MgF2 is 6.6 × 10-9.
A)1.3 × 10-7 M
B)6.6 × 10-9 M
C)6.6 × 10-8 M
D)1.6 × 10-7 M
E)6.6 × 10-7 M
A)1.3 × 10-7 M
B)6.6 × 10-9 M
C)6.6 × 10-8 M
D)1.6 × 10-7 M
E)6.6 × 10-7 M

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
48
Calculate the minimum concentration of Ag+ ion that must be added to (or built up in)a 0.140 M Na2CrO4 solution, in order to initiate a precipitation of silver chromate. The Ksp of Ag2CrO4 is 1.2 × 10-12.Hint: Use an ICE table to help organize your given information and remember to account for the presence of a common ion.
A)4.8 × 10-9 mol L-1
B)2.9 × 10-6 mol L-1
C)2.0 × 10-6 mol L-1
D)9.5 × 10-7 mol L-1
E)1.4 × 10-6 mol L-1
A)4.8 × 10-9 mol L-1
B)2.9 × 10-6 mol L-1
C)2.0 × 10-6 mol L-1
D)9.5 × 10-7 mol L-1
E)1.4 × 10-6 mol L-1

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
49
The solubility product for Ag3PO4 is 2.8 × 10-18. What is the solubility of silver phosphate in a solution which also contains 0.10 moles of silver nitrate per liter?Hint: Use an ICE table to help organize your given information and remember to account for the presence of a common ion.
A)4.4 × 10-4 mol L-1
B)4.4 × 10-15 mol L-1
C)2.8 × 10-15 mol L-1
D)3.6 × 10-16 mol L-1
E)2.8 × 10-13 mol L-1
A)4.4 × 10-4 mol L-1
B)4.4 × 10-15 mol L-1
C)2.8 × 10-15 mol L-1
D)3.6 × 10-16 mol L-1
E)2.8 × 10-13 mol L-1

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
50
Will a precipitate form when 20.0 mL of 1.8 × 10-3 M Pb(NO3)2 is added to 30.0 mL of5.0 × 10-4 M Na2SO4? The Ksp of (PbSO4)is 6.3 × 10-7.Hint: Find the concentration of each ion in the insoluble compound, and use those to find Q.
A)no, because the ion product, Q < Ksp
B)no, because the ion product, Q > Ksp
C)yes, because the ion product, Q < Ksp
D)yes, because the ion product, Q > Ksp
E)no, because Ksp is less than the ion product
A)no, because the ion product, Q < Ksp
B)no, because the ion product, Q > Ksp
C)yes, because the ion product, Q < Ksp
D)yes, because the ion product, Q > Ksp
E)no, because Ksp is less than the ion product

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
51
Will a precipitate of MgF2 form when 300 mL of 1.1 × 10-3 M MgCl2 solution is added to 500 mL of 1.2 × 10-3 M NaF? The Ksp of MgF2 is 6.9 × 10-9.Hint: Find the concentration of each ion in the insoluble compound, and use those to find Q.
A)yes, because the ion product, Q > Ksp
B)no, because the ion product, Q < Ksp
C)no, because the ion product, Q = Ksp
D)yes, because the ion product, Q < Ksp
E)no, because the ion product, Q > Ksp
A)yes, because the ion product, Q > Ksp
B)no, because the ion product, Q < Ksp
C)no, because the ion product, Q = Ksp
D)yes, because the ion product, Q < Ksp
E)no, because the ion product, Q > Ksp

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
52
The pH of a saturated solution of cerium (III)hydroxide in water is 9.20. Use this data to calculate a value for the solubility product constant of cerium(III)hydroxide.Hint: Use an ICE table to help organize your given information and use pH to find the equilibrium [OH-].
A)2.5 × 10-10
B)8.4 × 10-11
C)4.0 × 10-19
D)2.1 × 10-20
E)6.3 × 10-20
A)2.5 × 10-10
B)8.4 × 10-11
C)4.0 × 10-19
D)2.1 × 10-20
E)6.3 × 10-20

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
53
During an experiment, 300 mL of 2.0 × 10-5 M AgNO3 is going to be added to 200 mL of 2.5 × 10-9 M NaI. Will a precipitate form? What is the precipitate? The Ksp forAgI is 8.3 × 10-17.Hint: Find the concentration of each ion and use those concentrations to find Q for any insoluble compounds that might form.
A)yes, the ppt is AgNO3(s)
B)yes, the ppt is NaNO3(s)
C)yes, the ppt is NaI(s)
D)yes, the ppt is AgI(s)
E)no
A)yes, the ppt is AgNO3(s)
B)yes, the ppt is NaNO3(s)
C)yes, the ppt is NaI(s)
D)yes, the ppt is AgI(s)
E)no

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
54
For PbCl2, Ksp = 1.7 × 10-5. Will a precipitate of PbCl2 form when 200 mL of 3.0 × 10-2 M Pb(NO3)2 solution is added to 300 mL of 5.0 × 10-2 M KCl?Hint: Find the concentration of each ion in the insoluble compound, and use those to find Q.
A)yes, the ion product, Q > Ksp
B)no, the ion product, Q < Ksp
C)no, the ion product, Q = Ksp
D)yes, the ion product, Q < Ksp
E)no, because the ion product, Q > Ksp
A)yes, the ion product, Q > Ksp
B)no, the ion product, Q < Ksp
C)no, the ion product, Q = Ksp
D)yes, the ion product, Q < Ksp
E)no, because the ion product, Q > Ksp

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
55
Methylamine, CH3NH2, is a weak molecular base with a value of 4.4 × 10-4 for Kb. An aqueous solution contains 0.200 M CH3NH2 and 0.400 M CH3NH3Cl per liter as the only solutes. If the Ksp of Mg(OH)2 is 7.1 × 10-12, what is the maximum [Mg2+] that can coexist with these solutes in the solution?Hint: Use the Henderson-Hasselbalch formula to find the pOH of the solution, and then use that to find [OH-].
A)8.1 × 10-9
B)3.2 × 10-8
C)9.2 × 10-6
D)1.5 × 10-4
E)3.7 × 10-1
A)8.1 × 10-9
B)3.2 × 10-8
C)9.2 × 10-6
D)1.5 × 10-4
E)3.7 × 10-1

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
56
Methylamine, CH3NH2, is a weak molecular base with a value of 4.4 × 10-4 for Kb. An aqueous solution contains 0.200 M CH3NH2 and 0.300 M CH3NH3Cl per liter as the only solutes. If the Ksp of Fe(OH)2 is 7.9 × 10-16, what is the maximum [Fe2+] that can coexist with these solutes in the solution?Hint: Use the Henderson-Hasselbalch formula to find the pOH of the solution, and then use that to find [OH-].
A)1.8 × 10-12
B)9.0 × 10-12
C)1.8 × 10-9
D)9.2 × 10-9
E)9.2 × 10-6
A)1.8 × 10-12
B)9.0 × 10-12
C)1.8 × 10-9
D)9.2 × 10-9
E)9.2 × 10-6

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
57
Dimethylamine, (CH3)2NH, is a weak molecular base with a value of 9.6 × 10-4.for Kb. An aqueous solution contains 0.350 M (CH3)2NH and 0.250 M (CH3)2NH2Cl per liter as the only solutes. If the Ksp of Mg(OH)2 is 7.1 × 10-12, what is the maximum [Mg2+] that can coexist with these solutes in the solution?Hint: Use the Henderson-Hasselbalch formula to find the pOH of the solution, and then use that to find [OH-].
A)1.7 × 10-7
B)3.9 × 10-6
C)7.7 × 10-6
D)1.5 × 10-5
E)4.9 × 10-3
A)1.7 × 10-7
B)3.9 × 10-6
C)7.7 × 10-6
D)1.5 × 10-5
E)4.9 × 10-3

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following would decrease the concentration of Pb+2 ionized in a solution of PbI2?
A)adding a solution of KI
B)adding a solution of Pb(NO3)2
C)changing the pH of the solution
D)addition of a catalyst
E)adding a solution of NaNO3
A)adding a solution of KI
B)adding a solution of Pb(NO3)2
C)changing the pH of the solution
D)addition of a catalyst
E)adding a solution of NaNO3

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
59
Zinc carbonate, a slightly soluble substance, is most soluble in which of the following solvents?
A)water
B)0.1 M ZnCl2(aq)
C)0.1 M NaOH(aq)
D)0.1 M HCl(aq)
E)0.2 M Na2CO3(aq)
A)water
B)0.1 M ZnCl2(aq)
C)0.1 M NaOH(aq)
D)0.1 M HCl(aq)
E)0.2 M Na2CO3(aq)

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
60
The value of the solubility product constant for barium carbonate is 5.0 × 10-9 and that of barium chromate is 2.1 × 10-10. From this data, what is the value of Kc for the reaction below?  Hint: Combine reactions and Ksp values like all other reactions and equilibrium constants.
Hint: Combine reactions and Ksp values like all other reactions and equilibrium constants.
A)1.1 × 10-18
B)4.2 × 10-2
C)4.8 × 10-9
D)4.9
E)24
 Hint: Combine reactions and Ksp values like all other reactions and equilibrium constants.
Hint: Combine reactions and Ksp values like all other reactions and equilibrium constants.A)1.1 × 10-18
B)4.2 × 10-2
C)4.8 × 10-9
D)4.9
E)24

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
61
The value of the solubility product constant for silver carbonate is 8.5 × 10-12 and that of silver chromate is 1.1 × 10-12. From this data, what is the value of Kc for the reaction below?  Hint: Combine reactions and Ksp values like all other reactions and equilibrium constants.
Hint: Combine reactions and Ksp values like all other reactions and equilibrium constants.
A)9.6 × 10-12
B)1.3 × 10-1
C)1.1 × 1023
D)7.7
E)9.4 × 10-24
 Hint: Combine reactions and Ksp values like all other reactions and equilibrium constants.
Hint: Combine reactions and Ksp values like all other reactions and equilibrium constants.A)9.6 × 10-12
B)1.3 × 10-1
C)1.1 × 1023
D)7.7
E)9.4 × 10-24

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
62
The value of the solubility product constant for calcium oxalate is 2.3 × 10-9 and that of calcium sulfate is 4.9 × 10-5. From this data, what is the value of Kc for the reaction below?  Hint: Combine reactions and Ksp values like all other reactions and equilibrium constants.
Hint: Combine reactions and Ksp values like all other reactions and equilibrium constants.
A)1.1 × 10-13
B)4.7 × 10-5
C)8.9 × 1012
D)4.9 × 10-5
E)2.1 × 104
 Hint: Combine reactions and Ksp values like all other reactions and equilibrium constants.
Hint: Combine reactions and Ksp values like all other reactions and equilibrium constants.A)1.1 × 10-13
B)4.7 × 10-5
C)8.9 × 1012
D)4.9 × 10-5
E)2.1 × 104

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
63
Which solid would be more soluble in a strong acid solution than in pure water?
A)KCl
B)MgCl2
C)NaNO3
D)LiBr
E)ZnCO3
A)KCl
B)MgCl2
C)NaNO3
D)LiBr
E)ZnCO3

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
64
Which solid would be more soluble in a strong acid solution than in pure water?
A)NaCl
B)MgBr2
C)LiNO3
D)CaCO3
E)AgBr
A)NaCl
B)MgBr2
C)LiNO3
D)CaCO3
E)AgBr

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
65
Given the following information: 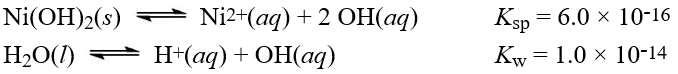 What is the equilibrium constant for the reaction below?
What is the equilibrium constant for the reaction below? 
A)1.7 × 1015
B)6.0 × 10-1
C)6.0 × 1012
D)1.7 × 10-13
E)3.3 × 101
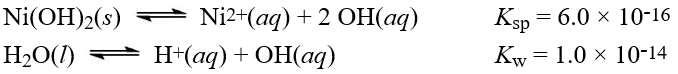 What is the equilibrium constant for the reaction below?
What is the equilibrium constant for the reaction below? 
A)1.7 × 1015
B)6.0 × 10-1
C)6.0 × 1012
D)1.7 × 10-13
E)3.3 × 101

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
66
Given the following information 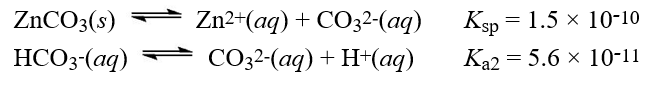 What is the equilibrium constant for the reaction below?
What is the equilibrium constant for the reaction below? 
A)8.4 × 10-21
B)3.7 × 10-1
C)1.2 × 1020
D)2.7
E)2.1 × 10-10
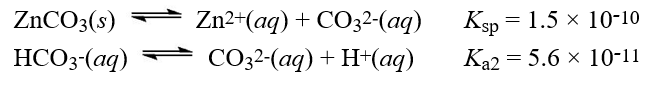 What is the equilibrium constant for the reaction below?
What is the equilibrium constant for the reaction below? 
A)8.4 × 10-21
B)3.7 × 10-1
C)1.2 × 1020
D)2.7
E)2.1 × 10-10

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
67
PbCO3, PbCl2, PbI2, and PbS are all only very slightly soluble in pure water. Which one (ones)should be significantly more soluble in acidic solution than in pure water?
A)PbI2, PbS and PbCO3
B)only PbCO3 and PbS
C)only PbCl2 and PbI2
D)only PbCO3
E)All four are significantly more soluble in acidic solution.
A)PbI2, PbS and PbCO3
B)only PbCO3 and PbS
C)only PbCl2 and PbI2
D)only PbCO3
E)All four are significantly more soluble in acidic solution.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
68
The group Zn(NO3)2, Zn(CO3)2, ZnCl2, and ZnS contains some salts which are only very slightly soluble in pure water. Of those salts, which one(s)should be significantly more soluble in acidic solution than in pure water?
A)Zn(NO3)2, Zn(CO3)2, and ZnCl2
B)Zn(NO3)2, and Zn(CO3)2
C)ZnCl2, and ZnS
D)Zn(CO3)2, and ZnCl2
E)Zn(CO3)2, and ZnS
A)Zn(NO3)2, Zn(CO3)2, and ZnCl2
B)Zn(NO3)2, and Zn(CO3)2
C)ZnCl2, and ZnS
D)Zn(CO3)2, and ZnCl2
E)Zn(CO3)2, and ZnS

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
69
The acid-insoluble sulfides and base-insoluble sulfides can be separated from each other by changing the pH of the aqueous solution that contains them. At low pH, the acid-insoluble sulfides will precipitate out. What role does the acid play in this process?
A)The H+ ion is produced by the dissolving sulfide, so the presence of an acid hinders the dissolution process.
B)The sulfide reacts with the H+ ion, forming the cation and H2S.
C)The sulfide reacts with any OH- ions present, forming S(OH)2 and the cation.
D)The sulfide forms a complex ion with the H+ provided by the acid.
E)The acid does not play a role in the dissolution process of metal sulfides.
A)The H+ ion is produced by the dissolving sulfide, so the presence of an acid hinders the dissolution process.
B)The sulfide reacts with the H+ ion, forming the cation and H2S.
C)The sulfide reacts with any OH- ions present, forming S(OH)2 and the cation.
D)The sulfide forms a complex ion with the H+ provided by the acid.
E)The acid does not play a role in the dissolution process of metal sulfides.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
70
The acid solubility product, Kspa, for PbS refers to which of the following reactions?
A)PbS(s)+ 2H2O(l) H+(aq)+ Pb(OH)2(s)+ HS-(l)
H+(aq)+ Pb(OH)2(s)+ HS-(l)
B)PbS(s)+ 2H2O(aq) H+(aq)+ Pb(OH)2(s)+ HS-(aq)
H+(aq)+ Pb(OH)2(s)+ HS-(aq)
C)PbS(s)+ 2H2O(l) H+(l)+ Pb(OH)2(s)
H+(l)+ Pb(OH)2(s)
D)PbS(s)+ 2H+(aq) Pb2+(aq)+ H2S(aq)
Pb2+(aq)+ H2S(aq)
E)PbS(s)+ 2H+(l) H+(aq)+ Pb(OH)2(s)+ HS-(aq)
H+(aq)+ Pb(OH)2(s)+ HS-(aq)
A)PbS(s)+ 2H2O(l)
 H+(aq)+ Pb(OH)2(s)+ HS-(l)
H+(aq)+ Pb(OH)2(s)+ HS-(l)B)PbS(s)+ 2H2O(aq)
 H+(aq)+ Pb(OH)2(s)+ HS-(aq)
H+(aq)+ Pb(OH)2(s)+ HS-(aq)C)PbS(s)+ 2H2O(l)
 H+(l)+ Pb(OH)2(s)
H+(l)+ Pb(OH)2(s)D)PbS(s)+ 2H+(aq)
 Pb2+(aq)+ H2S(aq)
Pb2+(aq)+ H2S(aq)E)PbS(s)+ 2H+(l)
 H+(aq)+ Pb(OH)2(s)+ HS-(aq)
H+(aq)+ Pb(OH)2(s)+ HS-(aq)
Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
71
A solution contains Ba2+ (1.0 × 10-3 M), Ca2+ (1.0 × 10-3 M)and K+ (1.0 × 10-3 M). Drops of a 5.0 × 10-1 M solution of NaF were added through a microburet until the [F-] in the solution mix reached 1.5 × 10-3 M. Should a precipitate form? If so, what is the precipitate? The Ksp values are BaF2: 1.0 × 10-6, CaF2: 5.3 × 10-9. (Neglect the slight change in volume resulting from the added NaF solution.)
A)a precipitate consisting of BaF2 only should form
B)a precipitate consisting of CaF2 and KF should form
C)a precipitate consisting of BaF2 and CaF2 should form
D)a precipitate consisting of CaF2 only should form
E)No precipitate should form.
A)a precipitate consisting of BaF2 only should form
B)a precipitate consisting of CaF2 and KF should form
C)a precipitate consisting of BaF2 and CaF2 should form
D)a precipitate consisting of CaF2 only should form
E)No precipitate should form.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
72
A solution contains Ba2+ (1.0 × 10-3 M), Ca2+ (1.0 × 10-3 M)and K+ (1.0 × 10-3 M). Drops of a 5.0 10-1 M solution of NaF were added through a microburet until the [F-] in the solution mix reached 3.0 × 10-3 M. Should a precipitate form? If so, what is the precipitate? The Ksp values are BaF2: 1.0 × 10-6, CaF2: 5.3 × 10-9. (Neglect the slight change in volume resulting from the added NaF solution.)
A)a precipitate consisting of BaF2 only should form
B)a precipitate consisting of CaF2 and KF should form
C)a precipitate consisting of BaF2 and CaF2 should form
D)a precipitate consisting of CaF2 only should form
E)No precipitate should form.
A)a precipitate consisting of BaF2 only should form
B)a precipitate consisting of CaF2 and KF should form
C)a precipitate consisting of BaF2 and CaF2 should form
D)a precipitate consisting of CaF2 only should form
E)No precipitate should form.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
73
A solution contains Ba2+ (1.0 × 10-3 M), Ca2+ (1.0 × 10-3 M)and K+ (1.0 × 10-3 M). Drops of a 5.0 × 10-1 M solution of NaF were added through a microburet until the [F-] in the solution mix reached 6.0 × 10-3 M. Should a precipitate form? If so, what is the precipitate? The Ksp values are BaF2: 1.0 × 10-6, CaF2: 5.3 × 10-9. (Neglect the slight change in volume resulting from the added NaF solution.)
A)a precipitate consisting of BaF2 only should form
B)a precipitate consisting of CaF2 and KF should form
C)a precipitate consisting of BaF2 and CaF2 should form
D)a precipitate consisting of CaF2 only should form
E)No precipitate should form.
A)a precipitate consisting of BaF2 only should form
B)a precipitate consisting of CaF2 and KF should form
C)a precipitate consisting of BaF2 and CaF2 should form
D)a precipitate consisting of CaF2 only should form
E)No precipitate should form.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
74
In an aqueous solution, silver ions can form Ag(NH3)2+(aq), in the presence of ammonia. In Ag(NH3)2+(aq),
A)Ag(NH3)2+(aq)is a ligand; NH3 is a donor atom; Ag+ is a ligand.
B)Ag(NH3)2+(aq)is a complex ion; NH3 is a ligand; Ag+ is a complex ion.
C)Ag(NH3)2+(aq)is a complex ion; NH3 is a donor atom; Ag+ is a ligand.
D)Ag(NH3)2+(aq)is an acceptor ion; NH3 is a ligand; Ag+ is a complex ion.
E)Ag(NH3)2+(aq)is a complex ion; NH3 is a ligand; Ag+ is an acceptor.
A)Ag(NH3)2+(aq)is a ligand; NH3 is a donor atom; Ag+ is a ligand.
B)Ag(NH3)2+(aq)is a complex ion; NH3 is a ligand; Ag+ is a complex ion.
C)Ag(NH3)2+(aq)is a complex ion; NH3 is a donor atom; Ag+ is a ligand.
D)Ag(NH3)2+(aq)is an acceptor ion; NH3 is a ligand; Ag+ is a complex ion.
E)Ag(NH3)2+(aq)is a complex ion; NH3 is a ligand; Ag+ is an acceptor.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
75
In an aqueous solution, copper ions can form the complex ion [Cu(H2O)6]+2(aq). In [Cu(H2O)6]+2(aq),
A)[Cu(H2O)6]+2(aq)is a complex ion; H2O is the solvent; Cu+2 is a ligand.
B)[Cu(H2O)6]+2(aq)is a complex ion; H2O is a ligand; Cu+2 is an acceptor.
C)[Cu(H2O)6]+2(aq)is a complex ion; H2O is a donor atom; Cu+2 is a ligand.
D)[Cu(H2O)6]+2(aq)is a ligand; H2O is an acceptor; Cu+2 is a complex ion.
E)[Cu(H2O)6]+2(aq)is a complex ion; H2O is a ligand; Cu+ is an acceptor.
A)[Cu(H2O)6]+2(aq)is a complex ion; H2O is the solvent; Cu+2 is a ligand.
B)[Cu(H2O)6]+2(aq)is a complex ion; H2O is a ligand; Cu+2 is an acceptor.
C)[Cu(H2O)6]+2(aq)is a complex ion; H2O is a donor atom; Cu+2 is a ligand.
D)[Cu(H2O)6]+2(aq)is a ligand; H2O is an acceptor; Cu+2 is a complex ion.
E)[Cu(H2O)6]+2(aq)is a complex ion; H2O is a ligand; Cu+ is an acceptor.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
76
Mercury ions (Hg2+)are very difficult to dissolve in a solution of water, or even a dilute solution of NaOH. But if ammonia is added to the water, mercury ions are more readily soluble. Which of the following best explains why this happens?
A)Mercury ions form complex ions with ammonia in solution, which aids the solution process.
B)Ammonia causes the mercury ions to become oxidized.
C)Ammonia causes the mercury ions to lose their charge.
D)Mercury is more soluble in a basic solution than in a neutral solution.
E)This process is still not fully understood by chemist.
A)Mercury ions form complex ions with ammonia in solution, which aids the solution process.
B)Ammonia causes the mercury ions to become oxidized.
C)Ammonia causes the mercury ions to lose their charge.
D)Mercury is more soluble in a basic solution than in a neutral solution.
E)This process is still not fully understood by chemist.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
77
The formation constant for the bis(thiosulfato)argentate(I)ion is 2.0 × 1013, while the solubility product constant for silver bromide is 5.0 × 10-13. What would be the equilibrium constant for the reaction below?AgBr(s)+ 2 S2O32-(aq)  Ag(S2O3)23-(aq)+ Br-(aq)
Ag(S2O3)23-(aq)+ Br-(aq)
A)2.0 × 1013
B)5.0 × 10-1
C)4.0 × 1025
D)5.0 × 10-13
E)10
 Ag(S2O3)23-(aq)+ Br-(aq)
Ag(S2O3)23-(aq)+ Br-(aq)A)2.0 × 1013
B)5.0 × 10-1
C)4.0 × 1025
D)5.0 × 10-13
E)10

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
78
A precipitate will form when a solution containing a cation and a solution containing an anion are mixed if the ________ exceeds the value of the ________.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
79
Addition of a common ion to a solution of a slightly soluble salt will ________ the solubility of the slightly soluble salt.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
80
The pH of a saturated solution of Mg(OH)2, whose Ksp is 7.1 × 10-12, is ________.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck


