Deck 14: Chemical Kinetics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/151
Play
Full screen (f)
Deck 14: Chemical Kinetics
1
Which of the following is a homogeneous reaction?
A)dissolving salt in water
B)neutralizing aqueous acid with solid sodium hydroxide
C)iron rusting
D)phosphorus igniting in air
E)titration of an unknown base with aqueous hydrochloric acid
A)dissolving salt in water
B)neutralizing aqueous acid with solid sodium hydroxide
C)iron rusting
D)phosphorus igniting in air
E)titration of an unknown base with aqueous hydrochloric acid
titration of an unknown base with aqueous hydrochloric acid
2
The fact that granulated sugar dissolves faster than a sugar cube of the same mass can best be explained by which of the following statements?
A)The ability of reacting or interacting molecules to come in contact with each other is greater for granulated sugar.
B)Dissolving the sugar increases the temperature of the solution.
C)Sugar cubes are coated to slow the dissolving process.
D)The concentration of granulated sugar is higher than that of a sugar cube.
E)None of the above
A)The ability of reacting or interacting molecules to come in contact with each other is greater for granulated sugar.
B)Dissolving the sugar increases the temperature of the solution.
C)Sugar cubes are coated to slow the dissolving process.
D)The concentration of granulated sugar is higher than that of a sugar cube.
E)None of the above
The ability of reacting or interacting molecules to come in contact with each other is greater for granulated sugar.
3
Most heterogeneous reactions are slower than similar homogeneous reactions. Which of the following best explains why?
A)The temperature of the two phases is different.
B)Heterogeneous mixtures are not allowed to react, and therefore are heterogeneous.
C)Heterogeneous mixtures require the use of a catalyst.
D)The ability of reactants to come in contact with each other in heterogeneous mixtures is limited by the different phases.
E)The activation energy of heterogeneous reactions is always much higher.
A)The temperature of the two phases is different.
B)Heterogeneous mixtures are not allowed to react, and therefore are heterogeneous.
C)Heterogeneous mixtures require the use of a catalyst.
D)The ability of reactants to come in contact with each other in heterogeneous mixtures is limited by the different phases.
E)The activation energy of heterogeneous reactions is always much higher.
The ability of reactants to come in contact with each other in heterogeneous mixtures is limited by the different phases.
4
Nitrogen monoxide reacts with bromine at elevated temperatures, according to the equation:2NO(g)+ Br2(g) 2NOBr(g)In a certain reaction mixture, the rate of formation of NOBr(g)was 4.50 × 10-4 mol L-1 s-1. What was the rate of consumption of Br2(g)?
A)4.50 × 10-4 mol L-1 s-1
B)2.25 × 10-4 mol L-1 s-1
C)9.00 × 10-4 mol L-1 s-1
D)2.12 × 10-4 mol L-1 s-1
E)2.03 × 10-3 mol L-1 s-1
A)4.50 × 10-4 mol L-1 s-1
B)2.25 × 10-4 mol L-1 s-1
C)9.00 × 10-4 mol L-1 s-1
D)2.12 × 10-4 mol L-1 s-1
E)2.03 × 10-3 mol L-1 s-1

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
5
Nitrogen monoxide reacts with chlorine at high temperature, according to the equation: 2NO(g)+ Cl2(g) 2NOCl(g)In a certain reaction mixture, the rate of formation of NOCl(g)was 4.50 × 10-4 mol L-1 s-1. What was the rate of consumption of NO(g)?
A)4.50 × 10-4 mol L-1 s-1
B)2.25 × 10-4 mol L-1 s-1
C)9.00 × 10-4 mol L-1 s-1
D)2.12 × 10-4 mol L-1 s-1
E)2.03 × 10-3 mol L-1 s-1
A)4.50 × 10-4 mol L-1 s-1
B)2.25 × 10-4 mol L-1 s-1
C)9.00 × 10-4 mol L-1 s-1
D)2.12 × 10-4 mol L-1 s-1
E)2.03 × 10-3 mol L-1 s-1

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
6
In a reaction described by the equation: 2CH4O(g)+ 3O2(g) 2CO2(g)+ 4H2O(g),the rate of consumption of O2(g)is 0.400 mol L-1 s-1. What is the rate of formation of H2O(g)?
A)0.300 mol L-1 s-1
B)0.400 mol L-1 s-1
C)0.533 mol L-1 s-1
D)0.800 mol L-1 s-1
E)1.33 mol L-1 s-1
A)0.300 mol L-1 s-1
B)0.400 mol L-1 s-1
C)0.533 mol L-1 s-1
D)0.800 mol L-1 s-1
E)1.33 mol L-1 s-1

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
7
In a reaction described by the equation:2 CH4O(g)+ 3 O2(g) 2 CO2(g)+ 4 H2O(g),the rate of consumption of O2(g)is 0.400 mol L-1 s-1. What is the rate of consumption of CH4O(g)?
A)0.200 mol L-1 s-1
B)0.267 mol L-1 s-1
C)0.333 mol L-1 s-1
D)0.400 mol L-1 s-1
E)0.600 mol L-1 s-1
A)0.200 mol L-1 s-1
B)0.267 mol L-1 s-1
C)0.333 mol L-1 s-1
D)0.400 mol L-1 s-1
E)0.600 mol L-1 s-1

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
8
Cyclobutane, C4H8, decomposes as shown: C4H8(g) 2 C2H4(g). In a study of this reaction, the rate of consumption of C4H8 at a certain point was 4.50 × 10-4 mol L-1 s-1. What is the rate at which C2H4(g)is being generated at this point?
A)4.50 × 10-4 mol L-1 s-1
B)2.25 × 10-4 mol L-1 s-1
C)9.00 × 10-4 mol L-1 s-1
D)2.12 × 10-4 mol L-1 s-1
E)2.03 × 10-3 mol L-1 s-1
A)4.50 × 10-4 mol L-1 s-1
B)2.25 × 10-4 mol L-1 s-1
C)9.00 × 10-4 mol L-1 s-1
D)2.12 × 10-4 mol L-1 s-1
E)2.03 × 10-3 mol L-1 s-1

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
9
A 10-mm cube of copper metal is placed in 250 mL of 12 M nitric acid at 25°C and the reaction below occurs: Cu(s)+ 4H+(aq)+ 2NO3-(aq) Cu2+(aq)+ 2NO2(g)+ 2H2O(l)At a particular instant in time, nitrogen dioxide is being produced at the rate of 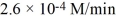 . At this same instant, what is the rate at which hydrogen ions are being consumed?
. At this same instant, what is the rate at which hydrogen ions are being consumed?
A)1.3 × 10-4 M/min
B)5.2 × 10-4 M/min
C)2.6 × 10-4 M/min
D)1.0 × 10-3 M/min
E)6.5 × 10-5 M/min
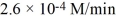 . At this same instant, what is the rate at which hydrogen ions are being consumed?
. At this same instant, what is the rate at which hydrogen ions are being consumed?A)1.3 × 10-4 M/min
B)5.2 × 10-4 M/min
C)2.6 × 10-4 M/min
D)1.0 × 10-3 M/min
E)6.5 × 10-5 M/min

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
10
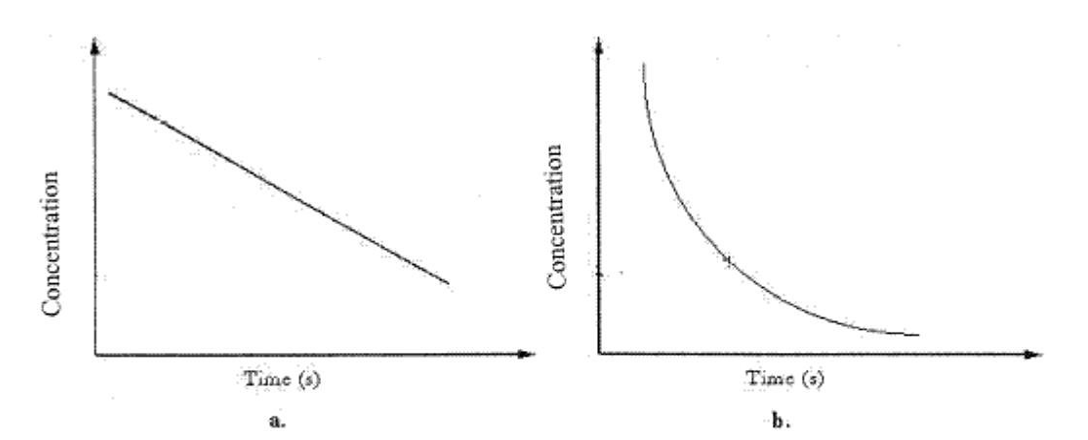 The average reaction rate over a time period in graph b can be found by
The average reaction rate over a time period in graph b can be found byA)taking the average of the rate every 100 s.
B)drawing a line tangent to the curve at the initial and final times, and then averaging.
C)drawing a line tangent to the curve at that point.
D)subtracting the final concentration from the initial concentration and dividing by the time interval.
E)subtracting the final time from the initial time and dividing by the concentration interval.

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
11
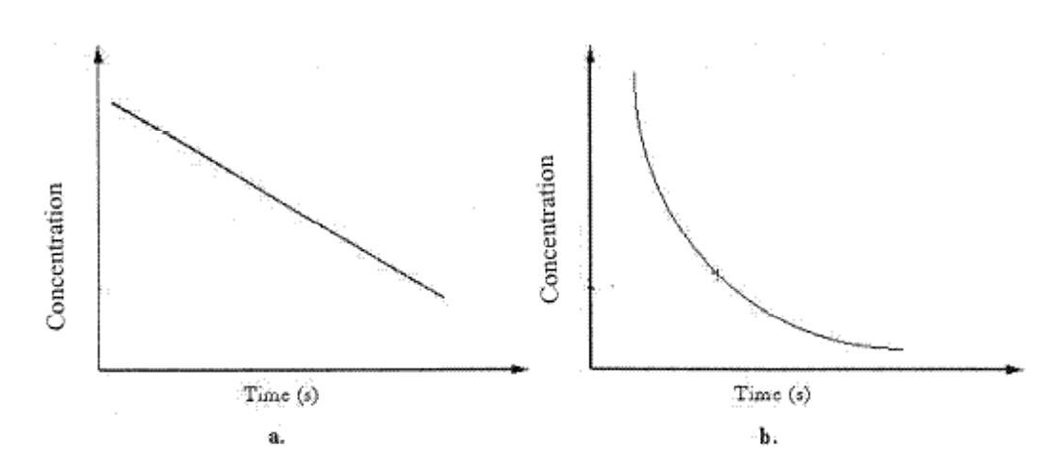 The instantaneous rate at any point in graph b can be found by
The instantaneous rate at any point in graph b can be found byA)taking the average of all rates found every 100 s.
B)drawing a line tangent to the curve at the initial and final times, and then averaging.
C)drawing a line tangent to the curve at that point.
D)subtracting the final concentration from the initial concentration and dividing by the time interval.
E)subtracting the final time from the initial time and dividing by the concentration.

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
12
The rate of a chemical reaction in solution can be measured in the units
A)L2 mol-1 s-1.
B)mol L-1 s-1.
C)s-2.
D)mol s L-1.
E)sec L-1 mol-1.
A)L2 mol-1 s-1.
B)mol L-1 s-1.
C)s-2.
D)mol s L-1.
E)sec L-1 mol-1.

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
13
A reaction has the rate law, rate = k[A][B]2. Which change will cause the greatest increase in the reaction rate?
A)decreasing both concentrations by half
B)doubling the concentration of B
C)quadrupling the concentration of A
D)tripling the concentration of B
E)doubling the concentration of A
A)decreasing both concentrations by half
B)doubling the concentration of B
C)quadrupling the concentration of A
D)tripling the concentration of B
E)doubling the concentration of A

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
14
A reaction has the rate law, rate = k[A]3[B]. Which change will cause the greatest increase in the reaction rate?
A)doubling the concentration of A and halving the concentration of B
B)quadrupling the concentration of B
C)tripling the concentration of A
D)doubling the concentration of A and tripling the concentration of B
E)tripling the concentration of B
A)doubling the concentration of A and halving the concentration of B
B)quadrupling the concentration of B
C)tripling the concentration of A
D)doubling the concentration of A and tripling the concentration of B
E)tripling the concentration of B

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
15
The reaction, 2NO(g)+ O2(g) 2NO2(g), was found to be first order in each of the two reactants and second order overall. The rate law is, therefore:
A)rate = k[NO]2
B)rate = k[NO][O2]
C)rate = k[NO2]2[NO]2[O2]½
D)rate = k[NO]2[O2]2
E)rate = k([NO][O2])2
A)rate = k[NO]2
B)rate = k[NO][O2]
C)rate = k[NO2]2[NO]2[O2]½
D)rate = k[NO]2[O2]2
E)rate = k([NO][O2])2

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
16
The reaction, NO2Cl(g)+ Cl(g) NO2(g)+ Cl2(g), was found to be first order in each of the two reactants and second order overall. The rate law is, therefore:
A)rate = k[NO2]2
B)rate = k[Cl]
C)rate = k[NO2Cl][Cl][NO2][Cl2]
D)rate = k[NO2Cl][Cl]
E)rate = k[NO2][ Cl2]
A)rate = k[NO2]2
B)rate = k[Cl]
C)rate = k[NO2Cl][Cl][NO2][Cl2]
D)rate = k[NO2Cl][Cl]
E)rate = k[NO2][ Cl2]

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
17
A reaction has the rate law: rate = k[A][B]2. What is the overall order of the reaction?
A)2nd
B)4th
C)1st
D)3rd
E)0
A)2nd
B)4th
C)1st
D)3rd
E)0

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
18
A reaction has the rate law, rate = k[A][B]3. What is the overall order of the reaction?
A)0
B)4th
C)1st
D)3rd
E)2nd
A)0
B)4th
C)1st
D)3rd
E)2nd

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
19
The reaction 2NO (g)+ 2H2 (g) N2 (g)+ 2H2O (g)was found to follow the rate law:rate = k[NO]2[H2]. By what factor will the rate of reaction increase if the pressure of NO gas is increased from 2.0 atm to 3.0 atm? Assume all other conditions are held constant.
A)1.5
B)1
C)2.25
D)2
E)3
A)1.5
B)1
C)2.25
D)2
E)3

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
20
A reaction has the rate law: rate = k[A][B]2. What is the order of the reaction, with respect to B?
A)2nd
B)4th
C)1st
D)3rd
E)0
A)2nd
B)4th
C)1st
D)3rd
E)0

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
21
A reaction has the rate law: rate = k[A]3[B]. What is the order of the reaction, with respect to A?
A)2nd
B)4th
C)1st
D)3rd
E)0
A)2nd
B)4th
C)1st
D)3rd
E)0

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
22
For the reaction, 2 XO + O2 2 XO2, data obtained from measurement of the initial rate of reaction at varying concentrations are given below. ![<strong>For the reaction, 2 XO + O<sub>2</sub> \rarr 2 XO<sub>2</sub>, data obtained from measurement of the initial rate of reaction at varying concentrations are given below. The rate law is therefore:</strong> A)rate = k[XO]<sup>2</sup> [O<sub>2</sub>] B)rate = k[XO][O<sub>2</sub>]<sup>2</sup> C)rate = k[XO][O<sub>2</sub>] D)rate = k[XO]<sup>2</sup> [O<sub>2</sub>]<sup>2</sup> E)rate = k[XO]<sup>2</sup>/[O<sub>2</sub>]<sup>2</sup>](https://storage.examlex.com/TBW1039/11ee6e7b_ba86_9937_bf7f_5f03ef707087_TBW1039_00.jpg) The rate law is therefore:
The rate law is therefore:
A)rate = k[XO]2 [O2]
B)rate = k[XO][O2]2
C)rate = k[XO][O2]
D)rate = k[XO]2 [O2]2
E)rate = k[XO]2/[O2]2
![<strong>For the reaction, 2 XO + O<sub>2</sub> \rarr 2 XO<sub>2</sub>, data obtained from measurement of the initial rate of reaction at varying concentrations are given below. The rate law is therefore:</strong> A)rate = k[XO]<sup>2</sup> [O<sub>2</sub>] B)rate = k[XO][O<sub>2</sub>]<sup>2</sup> C)rate = k[XO][O<sub>2</sub>] D)rate = k[XO]<sup>2</sup> [O<sub>2</sub>]<sup>2</sup> E)rate = k[XO]<sup>2</sup>/[O<sub>2</sub>]<sup>2</sup>](https://storage.examlex.com/TBW1039/11ee6e7b_ba86_9937_bf7f_5f03ef707087_TBW1039_00.jpg) The rate law is therefore:
The rate law is therefore:A)rate = k[XO]2 [O2]
B)rate = k[XO][O2]2
C)rate = k[XO][O2]
D)rate = k[XO]2 [O2]2
E)rate = k[XO]2/[O2]2

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
23
The units of the rate constant for a particular reaction are min-1. What is the overall order of the reaction?
A)Zero
B)First
C)Second
D)Third
E)Fourth
A)Zero
B)First
C)Second
D)Third
E)Fourth

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
24
The units of the rate constant for a particular reaction are L mol-1 min-1. What is the overall order of the reaction?
A)Zero
B)First
C)Second
D)Third
E)Fourth
A)Zero
B)First
C)Second
D)Third
E)Fourth

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
25
Nitric oxide reacts with bromine at elevated temperatures according to the equation 2NO(g)+ Br2(g) 2 NOBr(g)The experimental rate law is rate = k[NO][Br2]. In a certain reaction mixture, the rate of formation of NOBr(g)was found to be 4.50 × 10-4 mol L-1 s-1. Which unit below is the correct unit for the rate constant?
A)mol L-1 s-1
B)s-1
C)mol2 L-2 s-1
D)L mol-1 s-1
E)L2 mol-2 s-1
A)mol L-1 s-1
B)s-1
C)mol2 L-2 s-1
D)L mol-1 s-1
E)L2 mol-2 s-1

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
26
For the reaction, 3B + C E + 2F, initial rate measurements were carried out and data from three trials are shown below: ![<strong>For the reaction, 3B + C \rarr E + 2F, initial rate measurements were carried out and data from three trials are shown below: The rate law, therefore, is</strong> A)rate = k[B]<sup>3</sup>[C] B)rate = k[B][C] C)rate = k[B]<sup>2</sup>[C]<sup>2</sup> D)rate = k[B]<sup>2</sup>[C] E)rate = k[B][C]<sup>2</sup>](https://storage.examlex.com/TBW1039/11ee6e7b_ba86_c048_bf7f_713bac960844_TBW1039_00.jpg) The rate law, therefore, is
The rate law, therefore, is
A)rate = k[B]3[C]
B)rate = k[B][C]
C)rate = k[B]2[C]2
D)rate = k[B]2[C]
E)rate = k[B][C]2
![<strong>For the reaction, 3B + C \rarr E + 2F, initial rate measurements were carried out and data from three trials are shown below: The rate law, therefore, is</strong> A)rate = k[B]<sup>3</sup>[C] B)rate = k[B][C] C)rate = k[B]<sup>2</sup>[C]<sup>2</sup> D)rate = k[B]<sup>2</sup>[C] E)rate = k[B][C]<sup>2</sup>](https://storage.examlex.com/TBW1039/11ee6e7b_ba86_c048_bf7f_713bac960844_TBW1039_00.jpg) The rate law, therefore, is
The rate law, therefore, isA)rate = k[B]3[C]
B)rate = k[B][C]
C)rate = k[B]2[C]2
D)rate = k[B]2[C]
E)rate = k[B][C]2

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
27
Given the reaction, aA + bB dD + eE If we try rate = k[A]q[B]r for a generic rate law statement, which one of the statements below is false?
A)The exponents q and r are often integers.
B)The exponent q and r must be determined experimentally.
C)The exponents q and r are equal to the coefficients a and b, respectively.
D)The overall order of the reaction is q + r.
E)The symbol k represents the rate constant.
A)The exponents q and r are often integers.
B)The exponent q and r must be determined experimentally.
C)The exponents q and r are equal to the coefficients a and b, respectively.
D)The overall order of the reaction is q + r.
E)The symbol k represents the rate constant.

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
28
The reaction, A + 2B products, was studied. A timer was started when reagents A and B were mixed, and stopped when a specific quantity of product C accumulated. The data contains initial amounts of reactants used and the time needed to reach the specific quantity of C. Based on the data below, we can conclude that 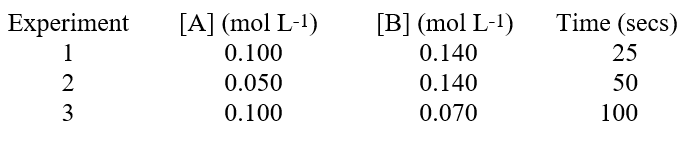 Hint: Remember reaction rate is proportional to 1/time.
Hint: Remember reaction rate is proportional to 1/time.
A)the reaction is first order with respect to substance A.
B)the reaction is zero order with respect to substance A.
C)the reaction is one-half order with respect to substance A.
D)the reaction is second order with respect to substance A.
E)the reaction is third order with respect to substance B
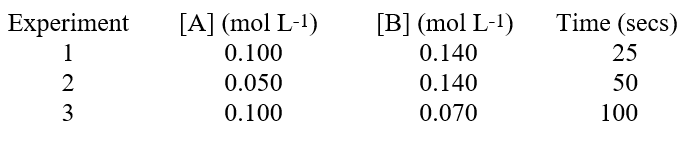 Hint: Remember reaction rate is proportional to 1/time.
Hint: Remember reaction rate is proportional to 1/time.A)the reaction is first order with respect to substance A.
B)the reaction is zero order with respect to substance A.
C)the reaction is one-half order with respect to substance A.
D)the reaction is second order with respect to substance A.
E)the reaction is third order with respect to substance B

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
29
For the reaction, 2M + 2N 2P + Q, studies on how the initial rate of the reaction varied with concentration were carried out. Some data is given below. ![<strong>For the reaction, 2M + 2N \rarr 2P + Q, studies on how the initial rate of the reaction varied with concentration were carried out. Some data is given below. </strong> A)the rate law is therefore: rate = k[N]<sup>2</sup> B)the rate law is therefore: rate = k[M][N]<sup>2</sup> C)the rate law is therefore: rate = k[M][N] D)the rate law is therefore: rate = k[M]<sup>2</sup> E)the rate law is therefore: rate = k[M]<sup>2</sup>[N]<sup>2</sup>](https://storage.examlex.com/TBW1039/11ee6e7b_ba86_e75a_bf7f_1d392ae69cae_TBW1039_00.jpg)
A)the rate law is therefore: rate = k[N]2
B)the rate law is therefore: rate = k[M][N]2
C)the rate law is therefore: rate = k[M][N]
D)the rate law is therefore: rate = k[M]2
E)the rate law is therefore: rate = k[M]2[N]2
![<strong>For the reaction, 2M + 2N \rarr 2P + Q, studies on how the initial rate of the reaction varied with concentration were carried out. Some data is given below. </strong> A)the rate law is therefore: rate = k[N]<sup>2</sup> B)the rate law is therefore: rate = k[M][N]<sup>2</sup> C)the rate law is therefore: rate = k[M][N] D)the rate law is therefore: rate = k[M]<sup>2</sup> E)the rate law is therefore: rate = k[M]<sup>2</sup>[N]<sup>2</sup>](https://storage.examlex.com/TBW1039/11ee6e7b_ba86_e75a_bf7f_1d392ae69cae_TBW1039_00.jpg)
A)the rate law is therefore: rate = k[N]2
B)the rate law is therefore: rate = k[M][N]2
C)the rate law is therefore: rate = k[M][N]
D)the rate law is therefore: rate = k[M]2
E)the rate law is therefore: rate = k[M]2[N]2

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
30
For the reaction, A + 2B C + 2D, the following data were obtained . ![<strong>For the reaction, A + 2B \rarr C + 2D, the following data were obtained . </strong> A)the rate law is therefore: rate = k[A][B]<sup>2</sup> B)the rate law is therefore: rate = k[B] C)the rate law is therefore: rate = k[A] D)the rate law is therefore: rate = k[A][B] E)the rate law is therefore: rate = k[A]<sup>2</sup>[B]](https://storage.examlex.com/TBW1039/11ee6e7b_ba86_e75b_bf7f_b1201302247e_TBW1039_00.jpg)
A)the rate law is therefore: rate = k[A][B]2
B)the rate law is therefore: rate = k[B]
C)the rate law is therefore: rate = k[A]
D)the rate law is therefore: rate = k[A][B]
E)the rate law is therefore: rate = k[A]2[B]
![<strong>For the reaction, A + 2B \rarr C + 2D, the following data were obtained . </strong> A)the rate law is therefore: rate = k[A][B]<sup>2</sup> B)the rate law is therefore: rate = k[B] C)the rate law is therefore: rate = k[A] D)the rate law is therefore: rate = k[A][B] E)the rate law is therefore: rate = k[A]<sup>2</sup>[B]](https://storage.examlex.com/TBW1039/11ee6e7b_ba86_e75b_bf7f_b1201302247e_TBW1039_00.jpg)
A)the rate law is therefore: rate = k[A][B]2
B)the rate law is therefore: rate = k[B]
C)the rate law is therefore: rate = k[A]
D)the rate law is therefore: rate = k[A][B]
E)the rate law is therefore: rate = k[A]2[B]

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
31
For the reaction, A + 2B C + 2D, some measurements of the initial rate of reaction at varying concentration gave the following data. ![<strong>For the reaction, A + 2B \rarr C + 2D, some measurements of the initial rate of reaction at varying concentration gave the following data. </strong> A)the rate law is therefore: rate = k[A]<sup>2</sup>[B] B)the rate law is therefore: rate = k[A][B]<sup>2</sup> C)the rate law is therefore: rate = k[A]<sup>2</sup>[B]<sup>2</sup> D)the rate law is therefore: rate = k[A][B] E)the rate law is therefore: rate = k[A][B]<sup>3</sup>](https://storage.examlex.com/TBW1039/11ee6e7b_ba86_e75c_bf7f_7f59f3f99cb6_TBW1039_00.jpg)
A)the rate law is therefore: rate = k[A]2[B]
B)the rate law is therefore: rate = k[A][B]2
C)the rate law is therefore: rate = k[A]2[B]2
D)the rate law is therefore: rate = k[A][B]
E)the rate law is therefore: rate = k[A][B]3
![<strong>For the reaction, A + 2B \rarr C + 2D, some measurements of the initial rate of reaction at varying concentration gave the following data. </strong> A)the rate law is therefore: rate = k[A]<sup>2</sup>[B] B)the rate law is therefore: rate = k[A][B]<sup>2</sup> C)the rate law is therefore: rate = k[A]<sup>2</sup>[B]<sup>2</sup> D)the rate law is therefore: rate = k[A][B] E)the rate law is therefore: rate = k[A][B]<sup>3</sup>](https://storage.examlex.com/TBW1039/11ee6e7b_ba86_e75c_bf7f_7f59f3f99cb6_TBW1039_00.jpg)
A)the rate law is therefore: rate = k[A]2[B]
B)the rate law is therefore: rate = k[A][B]2
C)the rate law is therefore: rate = k[A]2[B]2
D)the rate law is therefore: rate = k[A][B]
E)the rate law is therefore: rate = k[A][B]3

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
32
The reaction, 2A2X4(g) 2A2X3(g)+ X2(g), was found to be first order. The rate law, therefore, should be:
A)rate = k([A2X3]2[X2])/[A2X4]2
B)rate = k[A2X4]2
C)rate = k([A2X3][X2])/[A2X4]
D)rate = k[A2X4]
E)rate = k[A2X4]2/([A2X3]2[X2])
A)rate = k([A2X3]2[X2])/[A2X4]2
B)rate = k[A2X4]2
C)rate = k([A2X3][X2])/[A2X4]
D)rate = k[A2X4]
E)rate = k[A2X4]2/([A2X3]2[X2])

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
33
The data below were obtained in a study on how the rate of a reaction was affected by the concentration of its reactants. The data indicate: 
A)the order of the reaction with respect to C cannot be determined.
B)the reaction is second order with respect to C.
C)the reaction is zero order with respect to C.
D)the reaction is first order with respect to C.
E)the order of the reaction with respect to C is minus one.

A)the order of the reaction with respect to C cannot be determined.
B)the reaction is second order with respect to C.
C)the reaction is zero order with respect to C.
D)the reaction is first order with respect to C.
E)the order of the reaction with respect to C is minus one.

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
34
Given these data in a study on how the rate of a reaction was affected by the concentration of the reactants: 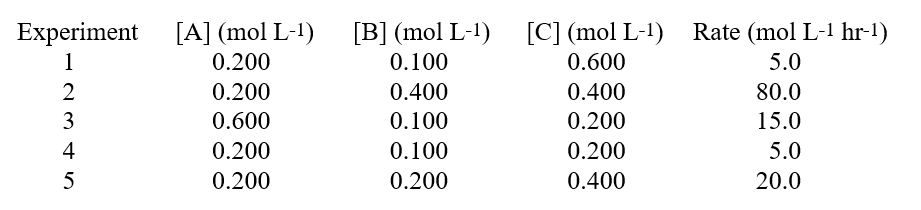 Hint: When you have three reactants, determine if any of them are 0 order.
Hint: When you have three reactants, determine if any of them are 0 order.
A)the reaction is zero order with respect to B.
B)the reaction is first order with respect to B.
C)the reaction order for B cannot be determined.
D)the reaction is second order with respect to B.
E)the reaction order for B is minus one.
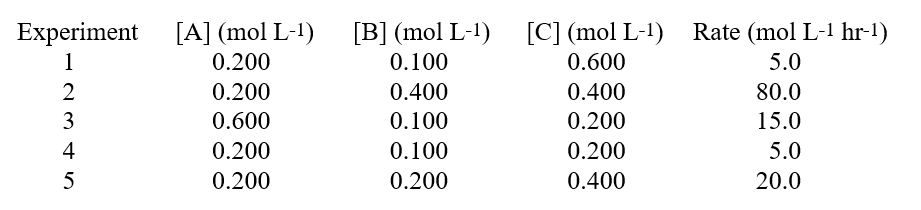 Hint: When you have three reactants, determine if any of them are 0 order.
Hint: When you have three reactants, determine if any of them are 0 order.A)the reaction is zero order with respect to B.
B)the reaction is first order with respect to B.
C)the reaction order for B cannot be determined.
D)the reaction is second order with respect to B.
E)the reaction order for B is minus one.

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
35
Given these data in a study on how the rate of a reaction was affected by the concentration of the reactants: ![<strong>Given these data in a study on how the rate of a reaction was affected by the concentration of the reactants: Hint: When you have three reactants, determine if any of them are 0 order.</strong> A)the reaction is first order with respect to A. B)the reaction is second order with respect to A. C)the reaction is zero order with respect to A. D)the reaction order for A is minus one (Rate proportional to 1/[A]). E)the reaction order for A cannot be determined from just this data alone.](https://storage.examlex.com/TBW1039/11ee6e7b_ba87_357f_bf7f_3fcd64ce621d_TBW1039_00.jpg) Hint: When you have three reactants, determine if any of them are 0 order.
Hint: When you have three reactants, determine if any of them are 0 order.
A)the reaction is first order with respect to A.
B)the reaction is second order with respect to A.
C)the reaction is zero order with respect to A.
D)the reaction order for A is minus one (Rate proportional to 1/[A]).
E)the reaction order for A cannot be determined from just this data alone.
![<strong>Given these data in a study on how the rate of a reaction was affected by the concentration of the reactants: Hint: When you have three reactants, determine if any of them are 0 order.</strong> A)the reaction is first order with respect to A. B)the reaction is second order with respect to A. C)the reaction is zero order with respect to A. D)the reaction order for A is minus one (Rate proportional to 1/[A]). E)the reaction order for A cannot be determined from just this data alone.](https://storage.examlex.com/TBW1039/11ee6e7b_ba87_357f_bf7f_3fcd64ce621d_TBW1039_00.jpg) Hint: When you have three reactants, determine if any of them are 0 order.
Hint: When you have three reactants, determine if any of them are 0 order.A)the reaction is first order with respect to A.
B)the reaction is second order with respect to A.
C)the reaction is zero order with respect to A.
D)the reaction order for A is minus one (Rate proportional to 1/[A]).
E)the reaction order for A cannot be determined from just this data alone.

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
36
Given these data in a study on how the rate of a reaction was affected by the concentration of the reactants, what is the numerical value of the rate constant, (k), for this reaction (where units match the data)?  Hint: When you have three reactants, determine if any of them are 0 order.
Hint: When you have three reactants, determine if any of them are 0 order.
A)2083
B)694
C)417
D)2500
E)83.3
 Hint: When you have three reactants, determine if any of them are 0 order.
Hint: When you have three reactants, determine if any of them are 0 order.A)2083
B)694
C)417
D)2500
E)83.3

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
37
What is the order of the reaction with respect to reactant A?Determining A Rate Law From Experimental Data The following data was found for the reaction:A + B Products 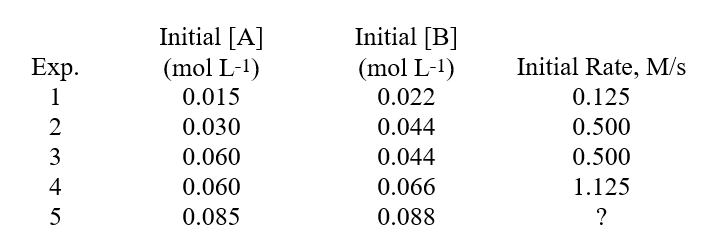
A)Zero
B)First
C)Second
D)Third
E)Fourth

A)Zero
B)First
C)Second
D)Third
E)Fourth

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
38
What is the order of the reaction with respect to reactant B? Determining A Rate Law From Experimental Data The following data was found for the reactionA + B Products 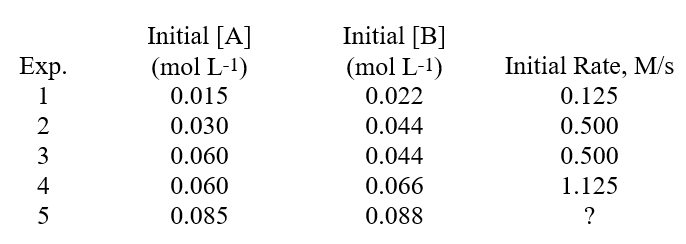
A)Zero
B)First
C)Second
D)Third
E)Fourth

A)Zero
B)First
C)Second
D)Third
E)Fourth

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
39
The data shown below was determined for the reaction A + B Products.Which of the following is the correct rate law for this reaction? ![<strong>The data shown below was determined for the reaction A + B \rarr Products.Which of the following is the correct rate law for this reaction? </strong> A)rate = k[A][B] B)rate = k[A] C)rate = k[B]<sup>2</sup> D)rate = k[B] E)rate = k[A][B]<sup>2</sup>](https://storage.examlex.com/TBW1039/11ee6e7b_ba87_3583_bf7f_85f5efc79c38_TBW1039_00.jpg)
A)rate = k[A][B]
B)rate = k[A]
C)rate = k[B]2
D)rate = k[B]
E)rate = k[A][B]2
![<strong>The data shown below was determined for the reaction A + B \rarr Products.Which of the following is the correct rate law for this reaction? </strong> A)rate = k[A][B] B)rate = k[A] C)rate = k[B]<sup>2</sup> D)rate = k[B] E)rate = k[A][B]<sup>2</sup>](https://storage.examlex.com/TBW1039/11ee6e7b_ba87_3583_bf7f_85f5efc79c38_TBW1039_00.jpg)
A)rate = k[A][B]
B)rate = k[A]
C)rate = k[B]2
D)rate = k[B]
E)rate = k[A][B]2

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
40
The data shown below was determined for the reaction A + B Products. What is the numerical value of the rate constant, (k), for this reaction? 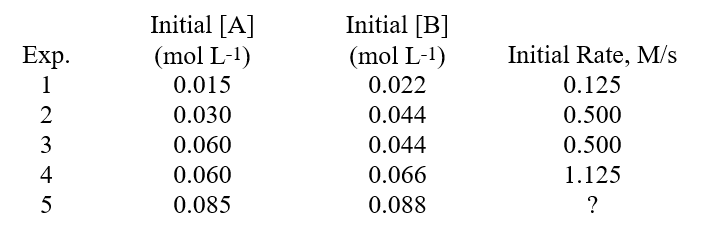
A)258.2
B)378.8
C)1.72 × 104
D)8.33
E)1.125

A)258.2
B)378.8
C)1.72 × 104
D)8.33
E)1.125

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
41
The data shown below was determined for the reaction A + B Products.What is the missing value in the table, for the initial rate of the reaction in Experiment 5? 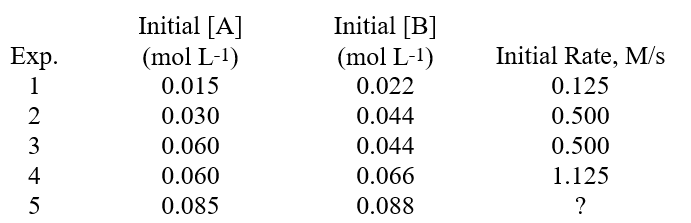
A)1.125
B)0.500
C)2.83
D)1.53
E)2.00

A)1.125
B)0.500
C)2.83
D)1.53
E)2.00

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
42
The half-life of a chemical reaction was found to be independent of the quantity of reactant which was employed. The reaction is, therefore,
A)possibly first order.
B)definitely first order.
C)zero order.
D)possibly second order.
E)definitely second order.
A)possibly first order.
B)definitely first order.
C)zero order.
D)possibly second order.
E)definitely second order.

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
43
If a reaction involving a single reactant is first order, with a rate constant of 4.50 × 10-2 s-1, how much time is required for 75.0% of the initial quantity of reactant to be used up?
A)16.7 seconds
B)30.9 seconds
C)23.1 seconds
D)25.3 seconds
E)11.6 seconds
A)16.7 seconds
B)30.9 seconds
C)23.1 seconds
D)25.3 seconds
E)11.6 seconds

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
44
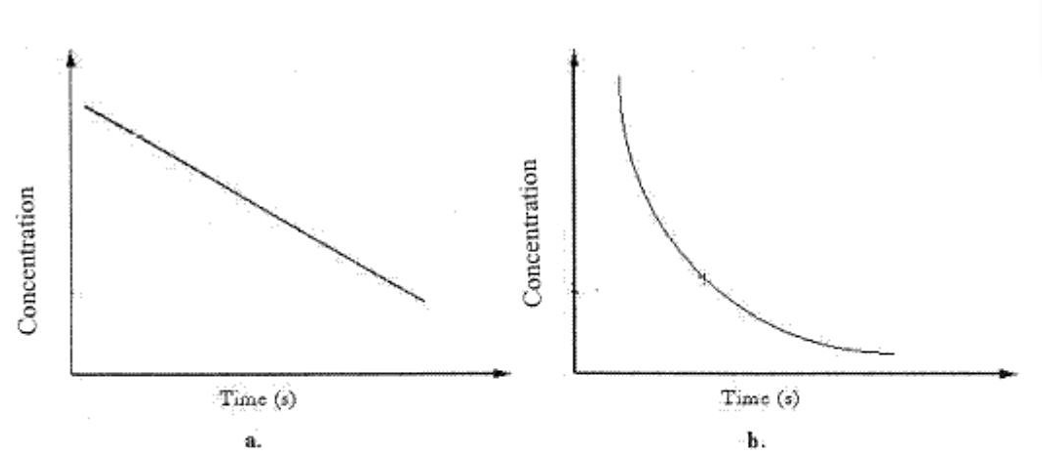 Graph b can best be described as
Graph b can best be described asA)Zero-order rate process.
B)First-order rate process.
C)Second-order rate process.
D)B or C
E)A or C

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
45
![<strong> We should determine the reaction order from graph b by</strong> A)plotting [A] vs. time. B)plotting 1/[A] vs. time. C)plotting ln[A] vs. time. D)plotting the square root of concentration vs. time. E)B and C](https://storage.examlex.com/TBW1039/11ee6e7b_ba87_83a7_bf7f_05330bf28ff6_TBW1039_00.jpg) We should determine the reaction order from graph b by
We should determine the reaction order from graph b byA)plotting [A] vs. time.
B)plotting 1/[A] vs. time.
C)plotting ln[A] vs. time.
D)plotting the square root of concentration vs. time.
E)B and C

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
46
Concentration vs. time data for a first-order reaction is shown in the graph below. What should be done to determine the half-life of the reaction? ![<strong>Concentration vs. time data for a first-order reaction is shown in the graph below. What should be done to determine the half-life of the reaction? </strong> A)Multiply [A] by k B)Plot 1/[A] vs. time C)Plot ln[A] vs. time D)Plot the square root of concentration vs. time E)B and C](https://storage.examlex.com/TBW1039/11ee6e7b_ba87_83a8_bf7f_a347f002d72d_TBW1039_00.jpg)
A)Multiply [A] by k
B)Plot 1/[A] vs. time
C)Plot ln[A] vs. time
D)Plot the square root of concentration vs. time
E)B and C
![<strong>Concentration vs. time data for a first-order reaction is shown in the graph below. What should be done to determine the half-life of the reaction? </strong> A)Multiply [A] by k B)Plot 1/[A] vs. time C)Plot ln[A] vs. time D)Plot the square root of concentration vs. time E)B and C](https://storage.examlex.com/TBW1039/11ee6e7b_ba87_83a8_bf7f_a347f002d72d_TBW1039_00.jpg)
A)Multiply [A] by k
B)Plot 1/[A] vs. time
C)Plot ln[A] vs. time
D)Plot the square root of concentration vs. time
E)B and C

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
47
The initial concentration of a reactant in a first-order reaction is 0.620 M. What will be its concentration after 3 half-lives have passed?
A)0.0865 M
B)0.0775 M
C)0.310 M
D)0.207 M
E)0.103 M
A)0.0865 M
B)0.0775 M
C)0.310 M
D)0.207 M
E)0.103 M

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
48
The initial concentration of a reactant in a first-order reaction is 0.860 M. What will be its concentration after 4 half-lives have passed?
A)0.215 M
B)0.0538 M
C)0.250 M
D)0.125 M
E)0.108 M
A)0.215 M
B)0.0538 M
C)0.250 M
D)0.125 M
E)0.108 M

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
49
The reaction A ? Products is a zero-order reaction with a rate constant of 4.81 × 10-3 M s-1. If the reaction starts with a 33.1 M concentration of A, at what time will the concentration of A be 24.1 M?
A)43.7 min
B)72.8 min
C)661 min
D)1870 min
E)31.2 min
A)43.7 min
B)72.8 min
C)661 min
D)1870 min
E)31.2 min

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
50
In a first-order reaction with only one reagent, the reaction was started with a concentration of reactant equal to 0.0800 M. After exactly two hours, the concentration had fallen to 0.0400 M. What is the molarity after exactly four hours?
A)0.0000 M
B)0.0100 M
C)0.0150 M
D)0.0200 M
E)0.0300 M
A)0.0000 M
B)0.0100 M
C)0.0150 M
D)0.0200 M
E)0.0300 M

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
51
The decomposition of an aldehyde solution in carbon tetrachloride is a first-order reaction with a rate constant of 1.20 × 10-3 min-1. If we start with [aldehyde] = 0.0500 M, what will the concentration of aldehyde be 150 minutes later?
A)0.00900 M
B)0.0418 M
C)0.00926 M
D)0.00499 M
E)0.000333 M
A)0.00900 M
B)0.0418 M
C)0.00926 M
D)0.00499 M
E)0.000333 M

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
52
The initial concentration of a reactant in a first order reaction is 0.620 M. What will be its concentration after 3 half-lives?
A)0.0865 M
B)0.310 M
C)0.0775 M
D)0.103 M
E)0.207 M
A)0.0865 M
B)0.310 M
C)0.0775 M
D)0.103 M
E)0.207 M

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
53
For the reaction, A B + C, the rate law is rate = k[A]. If it takes 80.0 seconds for 70.0% of a 10.0 gram sample of A to be transformed into products, what is the value of the rate constant?
A)0.00450 s-1
B)0.0290 s-1
C)0.00530 s-1
D)0.0150 s-1
E)5.40 s-1
A)0.00450 s-1
B)0.0290 s-1
C)0.00530 s-1
D)0.0150 s-1
E)5.40 s-1

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
54
For the reaction, A B + C, the rate law is rate = k[A] where k = 0.0175 s-1. How long will it take to reach 35.0 g from a 100.0 g sample?
A)1.00 min
B)7.77 min
C)0.0184 s
D)39.6 s
E)1.06 s
A)1.00 min
B)7.77 min
C)0.0184 s
D)39.6 s
E)1.06 s

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
55
The reaction of substance A with substance C was carefully studied under conditions where the [C] remained essentially constant. The graph of [A] vs. time gave a straight line while the graph of ln[A] vs. time and that of 1/[A] vs. time both gave curves.
A)The reaction is therefore zero order with respect to A.
B)The reaction is therefore one-half order with respect to A.
C)The reaction is therefore first order with respect to A.
D)The reaction is therefore second order with respect to A.
E)The reaction is therefore third order with respect to A.
A)The reaction is therefore zero order with respect to A.
B)The reaction is therefore one-half order with respect to A.
C)The reaction is therefore first order with respect to A.
D)The reaction is therefore second order with respect to A.
E)The reaction is therefore third order with respect to A.

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
56
A reaction is first-order overall. For a given sample, its initial rate is 0.0200 mol L-1 s-1, and 25.0 days later its rate dropped to 6.25 × 10-4 mol L-1 s-1. What is its half-life?
A)25.0 days
B)50.0 days
C)12.5 days
D)5.0 days
E)37.5 days
A)25.0 days
B)50.0 days
C)12.5 days
D)5.0 days
E)37.5 days

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
57
In a first-order reaction with only one reagent, the reaction was started with a concentration of reactant equal to 0.0800 M. After exactly two hours, the concentration had fallen to 0.0400 M. What is the molarity after exactly six hours?
A)0.0000 M
B)0.0100 M
C)0.0150 M
D)0.0200 M
E)0.0300 M
A)0.0000 M
B)0.0100 M
C)0.0150 M
D)0.0200 M
E)0.0300 M

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
58
The rate constant for a first-order decomposition reaction is 0.0111 min-1. What is the half-life of the reaction?
A)111 min
B)62.4 min
C)5000 sec
D)31.25 min
E)27.1 min
A)111 min
B)62.4 min
C)5000 sec
D)31.25 min
E)27.1 min

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
59
Given a reaction, 2A + B P, for which the rate law is rate = k[A]. Which equation or statement is true?
A)[A] = 1/kt
B)ln[A] = k/t
C)1/[A] = kt
D)The half-life is 0.693/k.
E)e[A] = kt
A)[A] = 1/kt
B)ln[A] = k/t
C)1/[A] = kt
D)The half-life is 0.693/k.
E)e[A] = kt

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
60
In a first-order reaction, what fraction of the reactant will remain after 4 half-lives?
A)1/16
B)1/8
C)1/9
D)1/4
E)1/3
A)1/16
B)1/8
C)1/9
D)1/4
E)1/3

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
61
In a first-order reaction, what fraction of the reactant will remain after 3 half-lives?
A)1/16
B)1/8
C)1/9
D)1/4
E)1/3
A)1/16
B)1/8
C)1/9
D)1/4
E)1/3

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
62
A first-order reaction has a rate constant of 0.00318 min1. The half-life of this reaction is, therefore,
A)94.7 minutes.
B)218 minutes.
C)31.4 minutes.
D)5.24 seconds.
E)68.6 minutes.
A)94.7 minutes.
B)218 minutes.
C)31.4 minutes.
D)5.24 seconds.
E)68.6 minutes.

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
63
For the reaction 2A + B Products The rate equation was found to be rate = k[A]2. If the rate constant was found to be 0.045 M-1 min-1 and the reaction started with an initial concentration of A equal to 0.50 M, what is the concentration of A after 30.0 minutes?
A)0.011 M
B)0.13 M
C)0.30 M
D)1.54 M
E)3.35 M
A)0.011 M
B)0.13 M
C)0.30 M
D)1.54 M
E)3.35 M

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
64
For the reaction 2A + B Products The rate equation was found to be rate = k[A]2. If the rate constant was found to be 0.035 M-1 min-1 and the reaction started with an initial concentration of A equal to 0.50 M, how long will it take for the concentration of A to reach 0.25 M?
A)4.2 sec
B)57.1 min
C)49.8 min
D)1.05 sec
E)53.5 min
A)4.2 sec
B)57.1 min
C)49.8 min
D)1.05 sec
E)53.5 min

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
65
The reaction A Products is a zero-order reaction with a rate constant of 2.56 × 10-3 M s-1. If the concentration of A is 75.6 M after 12.7 minutes, what was the initial concentration of A?
A)838 M
B)100 M
C)75.6 M
D)77.6 M
E)73.6 M
A)838 M
B)100 M
C)75.6 M
D)77.6 M
E)73.6 M

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
66
At a certain temperature the second-order reaction NOCl(g) NO(g)+ 1/2Cl2(g)is 50% complete after 6.23 hours when the initial concentration of NOCl is 4.78 mol/L. How long will it take for the reaction to be 75% complete?
A)18.7 hr
B)17.5 hr
C)8.2 hr
D)31.5 hr
E)12.5 hr.
A)18.7 hr
B)17.5 hr
C)8.2 hr
D)31.5 hr
E)12.5 hr.

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
67
Charcoal taken from the site of an ancient village was found to have a 14C to 12C ratio of one-third that is found in living plant life. Using this data, what is the age of the charcoal sample? The half-life of 14C is 5730 years.
A)12,300 yrs.
B)9,080 yrs.
C)6,950 yrs.
D)1,800 yrs.
E)2510 yrs.
A)12,300 yrs.
B)9,080 yrs.
C)6,950 yrs.
D)1,800 yrs.
E)2510 yrs.

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
68
For a first-order reaction with a single reactant, after 230.0 seconds, 10.0% of the reactant remains. The rate constant for the reaction is, therefore,
A)0.000640 s-1
B)0.0100 s-1
C)100 s-1
D)0.0510 s-1
E)0.0915 s-1
A)0.000640 s-1
B)0.0100 s-1
C)100 s-1
D)0.0510 s-1
E)0.0915 s-1

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
69
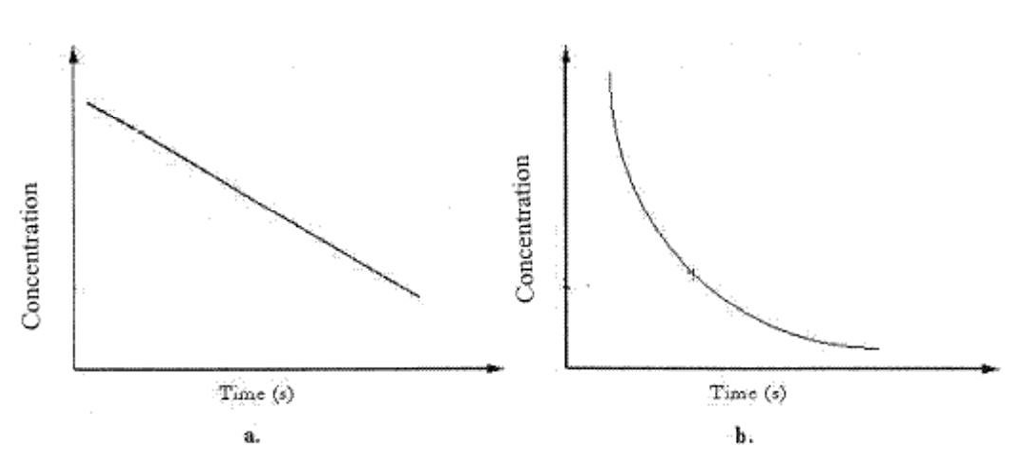 Graph a can best be described as a
Graph a can best be described as aA)Zero-order rate process.
B)First-order rate process.
C)Second-order rate process.
D)B or C
E)A or C

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
70
The reaction of substance A with substance C was carefully studied under conditions where the [C] remained essentially constant. The graphs of [A] vs. time and that of ln[A] vs. time both gave curves, but the graph of 1/[A] vs. time gave a straight line.
A)The reaction is therefore zero order with respect to A.
B)The reaction is therefore one-half order with respect to A.
C)The reaction is therefore first order with respect to A.
D)The reaction is therefore second order with respect to A.
E)The reaction is therefore third order with respect to A.
A)The reaction is therefore zero order with respect to A.
B)The reaction is therefore one-half order with respect to A.
C)The reaction is therefore first order with respect to A.
D)The reaction is therefore second order with respect to A.
E)The reaction is therefore third order with respect to A.

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
71
A sample of Peruvian corn was found to have a 14C to 12C ratio of 8.0 × 10-13. Similar corn samples taken present day have a 14C to 12C ratio of 1.2 × 10-12. Using this data, what is the age of the Peruvian corn sample?Hint: The half-life of 14C is 5730 years.
A)2.5 yrs.
B)15,700 yrs.
C)5,240 yrs.
D)1,470 yrs.
E)3,350 yrs.
A)2.5 yrs.
B)15,700 yrs.
C)5,240 yrs.
D)1,470 yrs.
E)3,350 yrs.

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
72
What two factors influence the effectiveness of molecular collisions in producing chemical change?
1. The molar masses of the reactants
2. The activation energy
3. The pressure
4. The orientation of the reactants
A)3 and 4
B)1 and 3
C)1 and 2
D)2 and 4
E)2 and 3
1. The molar masses of the reactants
2. The activation energy
3. The pressure
4. The orientation of the reactants
A)3 and 4
B)1 and 3
C)1 and 2
D)2 and 4
E)2 and 3

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
73
When there is an increase in temperature of a system the rate of reaction often increases. Which of the following best explains why this happens?
1. The concentrations increase with temperature.
2. The number of collisions with the sufficient kinetic energy increase with temperature.
3. The number of collisions per unit time increases with temperature.
4. The orientation of the molecules change with temperature.
A)3 and 4
B)1 and 3
C)1 and 2
D)2 and 4
E)2 and 3
1. The concentrations increase with temperature.
2. The number of collisions with the sufficient kinetic energy increase with temperature.
3. The number of collisions per unit time increases with temperature.
4. The orientation of the molecules change with temperature.
A)3 and 4
B)1 and 3
C)1 and 2
D)2 and 4
E)2 and 3

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
74
For a one step reaction, the activation energy for the forward reaction is 40.0 kJ mol-1, and the heat of reaction is 20.0 kJ mol-1. Which statement below is true?
A)The activation energy of the forward reaction would be affected to a greater extent than the activation energy of the reverse reaction by addition of a catalyst.
B)The value for the heat of reaction would be decreased by addition of a catalyst.
C)The reaction is endothermic.
D)The reverse reaction has a higher activation energy than the forward reaction.
E)The reaction rate would be decreased by an increase in temperature.
A)The activation energy of the forward reaction would be affected to a greater extent than the activation energy of the reverse reaction by addition of a catalyst.
B)The value for the heat of reaction would be decreased by addition of a catalyst.
C)The reaction is endothermic.
D)The reverse reaction has a higher activation energy than the forward reaction.
E)The reaction rate would be decreased by an increase in temperature.

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
75
For a one-step reaction, the activation energy for the forward reaction is 40.0 kJ mol-1, and the heat of reaction is 20.0 kJ mol-1. Calculate the activation energy for the reverse reaction.
A)+60.0 kJ mol-1
B)-20.0 kJ mol-1
C)-1200 kJ mol-1
D)+20.0 kJ mol-1
E)+1200 kJ mol-1
A)+60.0 kJ mol-1
B)-20.0 kJ mol-1
C)-1200 kJ mol-1
D)+20.0 kJ mol-1
E)+1200 kJ mol-1

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
76
The reaction progress for a given reaction is shown below. Which corresponds to the activation energy of the forward reaction, Ea? 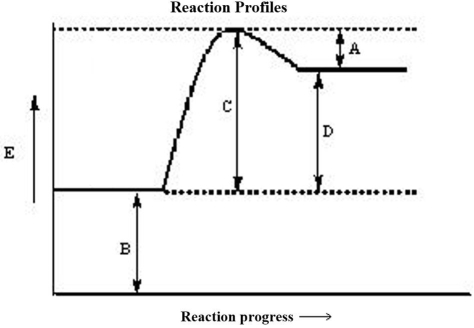
A)A
B)B
C)C
D)D
E)none of these

A)A
B)B
C)C
D)D
E)none of these

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
77
The reaction progress for a given reaction is shown below. Which corresponds to the overall energy change of the reaction, 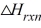 ? (The heat of reaction)
? (The heat of reaction) 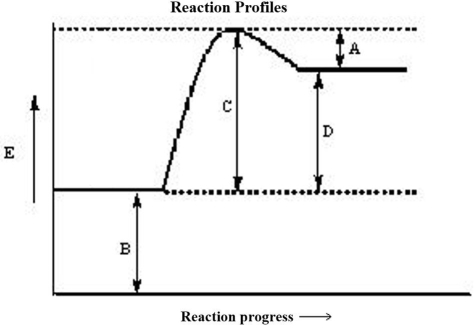
A)A
B)B
C)C
D)D
E)none of these
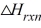 ? (The heat of reaction)
? (The heat of reaction) 
A)A
B)B
C)C
D)D
E)none of these

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
78
The reaction progress for a given reaction is shown below. The point on the diagram where the energy of the system is at a maximum is called the 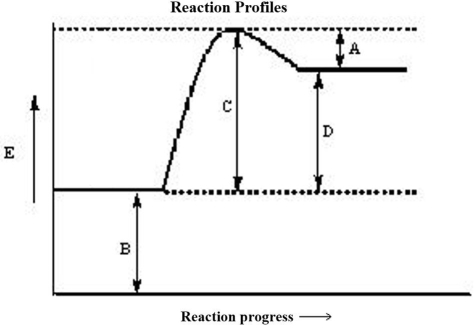
A)transition state.
B)critical point.
C)formation energy of the reactants.
D)formation energy of the products.
E)rate determining state.

A)transition state.
B)critical point.
C)formation energy of the reactants.
D)formation energy of the products.
E)rate determining state.

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
79
The reaction progress for a given reaction is shown below. Which corresponds to the activation energy of the reverse reaction, Ea? 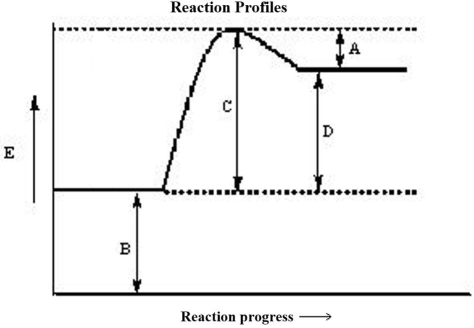
A)A
B)B
C)C
D)D
E)none of these

A)A
B)B
C)C
D)D
E)none of these

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck
80
The reaction A + 3B D + F was studied, and the following mechanism was determined 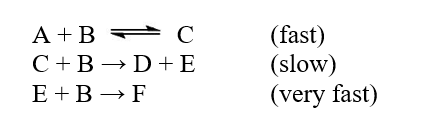 The step with largest activation energy is
The step with largest activation energy is
A)the first step.
B)the second step.
C)the third step.
D)None of the steps has an activation energy.
E)All of the steps have the same activation energy.
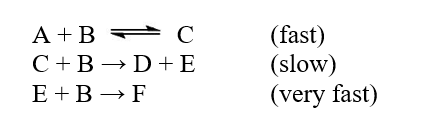 The step with largest activation energy is
The step with largest activation energy isA)the first step.
B)the second step.
C)the third step.
D)None of the steps has an activation energy.
E)All of the steps have the same activation energy.

Unlock Deck
Unlock for access to all 151 flashcards in this deck.
Unlock Deck
k this deck


