Deck 8: The Quantum Mechanical Atom
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/219
Play
Full screen (f)
Deck 8: The Quantum Mechanical Atom
1
The frequency of an electromagnetic wave is
A)the number of complete oscillations or cycles over a distance of one meter.
B)the number of complete oscillations or cycles in a one second time interval.
C)the distance between successive maxima in the wave in one complete cycle.
D)the number of complete oscillations or cycles over a distance of one centimeter.
E)the distance between successive nodes in the wave.
A)the number of complete oscillations or cycles over a distance of one meter.
B)the number of complete oscillations or cycles in a one second time interval.
C)the distance between successive maxima in the wave in one complete cycle.
D)the number of complete oscillations or cycles over a distance of one centimeter.
E)the distance between successive nodes in the wave.
the number of complete oscillations or cycles in a one second time interval.
2
The wavelength of an electromagnetic wave is
A)the number of complete oscillations or cycles over a distance of one meter.
B)the number of complete oscillations or cycles in a one second time interval.
C)the distance between successive maxima in the wave.
D)the number of complete oscillations or cycles over a distance of one centimeter.
E)the distance between a minimum and the nearest maximum in the oscillation.
A)the number of complete oscillations or cycles over a distance of one meter.
B)the number of complete oscillations or cycles in a one second time interval.
C)the distance between successive maxima in the wave.
D)the number of complete oscillations or cycles over a distance of one centimeter.
E)the distance between a minimum and the nearest maximum in the oscillation.
the distance between successive maxima in the wave.
3
What is the wavelength of electromagnetic radiation which has a frequency of 5.732 × 1014 s-1?
A)1.718 × 1023 m
B)1.912 × 106 m
C)5.234 × 10-7 m
D)523.4 m
E)5.819 × 10-15 nm
A)1.718 × 1023 m
B)1.912 × 106 m
C)5.234 × 10-7 m
D)523.4 m
E)5.819 × 10-15 nm
5.234 × 10-7 m
4
What is the wavelength of electromagnetic radiation which has a frequency of 4.464 × 1014 s-1?
A)1.338 ×1023 m
B)1.489 × 10-6 m
C)4.716 × 107 nm
D)6.720 × 102 nm
E)7.472 × 10-15 nm
A)1.338 ×1023 m
B)1.489 × 10-6 m
C)4.716 × 107 nm
D)6.720 × 102 nm
E)7.472 × 10-15 nm

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
5
What is the wavelength of electromagnetic radiation which has a frequency of 3.818 × 1014 s-1?
A)1145 nm
B)1.274 × 10-1 nm
C)1.274 × 10-7 m
D)7.858 × 10-7 nm
E)7.858 × 10-7 m
A)1145 nm
B)1.274 × 10-1 nm
C)1.274 × 10-7 m
D)7.858 × 10-7 nm
E)7.858 × 10-7 m

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
6
What is the wavelength of electromagnetic radiation which has a frequency of 6.282 × 1014 s-1?
A)1.883 × 1023 m
B)2.095 × 106 m
C)4.776 × 10-7 m
D)4.776 × 10-7 nm
E)530.9 nm
A)1.883 × 1023 m
B)2.095 × 106 m
C)4.776 × 10-7 m
D)4.776 × 10-7 nm
E)530.9 nm

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
7
Calculate the frequency of visible light having a wavelength of 464.1 nm.
A)139.1 s-1
B)1.548 × 10-6 s-1
C)1.548 × 10-15 s-1
D)6.464 × 1014 s-1
E)6.464 × 105 s-1
A)139.1 s-1
B)1.548 × 10-6 s-1
C)1.548 × 10-15 s-1
D)6.464 × 1014 s-1
E)6.464 × 105 s-1

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
8
Calculate the frequency of visible light having a wavelength of 589.3 nm.
A)176.7 s-1
B)1.966 × 10-15 s-1
C)1.391 × 10-11 s-1
D)5.091 × 1014 s-1
E)5.660 × 103 s-1
A)176.7 s-1
B)1.966 × 10-15 s-1
C)1.391 × 10-11 s-1
D)5.091 × 1014 s-1
E)5.660 × 103 s-1

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
9
A police radar unit is operating on a frequency of 9.527 gigahertz. What is the wavelength of the radiation being employed?
A)314.9 nm
B)314.9 m
C)3.149 cm
D)314.9 cm
E)31.78 m
A)314.9 nm
B)314.9 m
C)3.149 cm
D)314.9 cm
E)31.78 m

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
10
Calculate the frequency of visible light having a wavelength of 568.8 nm.
A)170.5 s-1
B)1.897 × 106 s-1
C)1.897 × 1015 s-1
D)5.274 × 10-9 s-1
E)5.274 × 1014 s-1
A)170.5 s-1
B)1.897 × 106 s-1
C)1.897 × 1015 s-1
D)5.274 × 10-9 s-1
E)5.274 × 1014 s-1

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
11
Calculate the frequency of visible light having a wavelength of 25.3 cm.
A)8.44 × 10-8 s-1
B)1.19 × 109 s-1
C)1.19 × 107 s-1
D)7.58 × 109 s-1
E)7.58 × 107 s-1
A)8.44 × 10-8 s-1
B)1.19 × 109 s-1
C)1.19 × 107 s-1
D)7.58 × 109 s-1
E)7.58 × 107 s-1

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
12
Which one of the following types of radiation has the lowest frequency?
A)radio waves
B)infrared radiation
C)microwave radiation
D)X-rays
E)ultraviolet rays
A)radio waves
B)infrared radiation
C)microwave radiation
D)X-rays
E)ultraviolet rays

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
13
Which radiation has the lowest frequency?
A)gamma rays
B)infrared radiation
C)microwave radiation
D)visible light rays
E)ultraviolet rays
A)gamma rays
B)infrared radiation
C)microwave radiation
D)visible light rays
E)ultraviolet rays

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
14
Which radiation has the highest frequency?
A)blue visible light
B)radio waves
C)infrared radiation
D)microwave radiation
E)red visible light
A)blue visible light
B)radio waves
C)infrared radiation
D)microwave radiation
E)red visible light

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
15
Which radiation has the highest frequency?
A)X-rays
B)ultraviolet rays
C)radio waves
D)microwave radiation
E)infrared radiation
A)X-rays
B)ultraviolet rays
C)radio waves
D)microwave radiation
E)infrared radiation

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
16
Which radiation has the shortest wavelength?
A)radio waves
B)infrared radiation
C)microwave radiation
D)ultraviolet rays
E)X-rays
A)radio waves
B)infrared radiation
C)microwave radiation
D)ultraviolet rays
E)X-rays

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
17
Which radiation has the shortest wavelength?
A)gamma rays
B)infrared radiation
C)microwave radiation
D)ultraviolet rays
E)visible light rays
A)gamma rays
B)infrared radiation
C)microwave radiation
D)ultraviolet rays
E)visible light rays

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
18
Which radiation has the shortest wavelength?
A)FM radio waves
B)infrared radiation
C)microwave radiation
D)ultraviolet rays
E)visible light rays
A)FM radio waves
B)infrared radiation
C)microwave radiation
D)ultraviolet rays
E)visible light rays

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
19
Which radiation has the longest wavelength?
A)gamma rays
B)green visible light rays
C)red visible light rays
D)ultraviolet rays
E)X-rays
A)gamma rays
B)green visible light rays
C)red visible light rays
D)ultraviolet rays
E)X-rays

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
20
Which radiation has the longest wavelength?
A)gamma rays
B)infrared radiation
C)microwave radiation
D)ultraviolet rays
E)red visible light rays
A)gamma rays
B)infrared radiation
C)microwave radiation
D)ultraviolet rays
E)red visible light rays

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
21
Which radiation has the longest wavelength?
A)infrared radiation
B)radio waves
C)microwave radiation
D)ultraviolet rays
E)X-rays
A)infrared radiation
B)radio waves
C)microwave radiation
D)ultraviolet rays
E)X-rays

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
22
What is the energy of one photon of microwave radiation with a wavelength of 0.158 m?
A)1.26 × 10-24 J
B)3.14 × 10-26 J
C)3.19 × 1025 J
D)3.49 × 10-43 J
E)7.15 × 1040 J
A)1.26 × 10-24 J
B)3.14 × 10-26 J
C)3.19 × 1025 J
D)3.49 × 10-43 J
E)7.15 × 1040 J

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
23
What is the energy of one photon of visible radiation with a wavelength of 464.1 nm?
A)1.03 × 10-48 J
B)2.10 × 1035 J
C)2.34 × 1011 J
D)4.28 × 10-19 J
E)4.28 × 10-12 J
A)1.03 × 10-48 J
B)2.10 × 1035 J
C)2.34 × 1011 J
D)4.28 × 10-19 J
E)4.28 × 10-12 J

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
24
What is the energy of one mole of photons of visible light having a wavelength of 486.1 nm?
A)12.4 kJ
B)2.46 × 10-4 J
C)2.46 × 105 J
D)6.17 × 1014 J
E)8.776.2 × 1025 J
A)12.4 kJ
B)2.46 × 10-4 J
C)2.46 × 105 J
D)6.17 × 1014 J
E)8.776.2 × 1025 J

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
25
The photoelectric effect
A)describes the interaction of light with a photograph.
B)describes how electrons interact with each other.
C)is the process in which electrons are ejected from certain material by photons.
D)is the process of light being emitted from an electrical wire.
E)describes the interactions of photons with a pane of glass.
A)describes the interaction of light with a photograph.
B)describes how electrons interact with each other.
C)is the process in which electrons are ejected from certain material by photons.
D)is the process of light being emitted from an electrical wire.
E)describes the interactions of photons with a pane of glass.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
26
What is the energy of one mole of photons of visible light having a wavelength of 4.89 × 102 nm?
A)1.48 × 1042 J
B)1.95 × 10-16 J
C)2.45 × 105 J
D)3.24 × 10-40 J
E)4.06 × 10-19 J
A)1.48 × 1042 J
B)1.95 × 10-16 J
C)2.45 × 105 J
D)3.24 × 10-40 J
E)4.06 × 10-19 J

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
27
What is the energy of one mole of photons associated with radiation that has a frequency of 6.336 × 1015 Hz?
A)2.528 × 106 J
B)3.882 × 1014 J
C)3.955 × 10-7 J
D)4.198 × 10-18 J
E)6.298 × 10-26 J
A)2.528 × 106 J
B)3.882 × 1014 J
C)3.955 × 10-7 J
D)4.198 × 10-18 J
E)6.298 × 10-26 J

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
28
What is the energy of one mole of photons associated with radiation that has a frequency of 3.818 × 1015 Hz?
A)1.045 × 10-25 J
B)1.524 × 106 J
C)2.530 × 10-18 J
D)6.564 × 10-7 J
E)9.568 × 1024 J
A)1.045 × 10-25 J
B)1.524 × 106 J
C)2.530 × 10-18 J
D)6.564 × 10-7 J
E)9.568 × 1024 J

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
29
What is the wavelength of radiation which has an energy of 3.371 × 10-19 joules per photon?
A)655.9 nm
B)152.5 nm
C)170.0 nm
D)589.3 nm
E)745.1 nm
A)655.9 nm
B)152.5 nm
C)170.0 nm
D)589.3 nm
E)745.1 nm

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
30
What is the wavelength of radiation which has an energy of 216.1 kJ per mole of photons?
A)655.9 nm
B)546.1 nm
C)108.8 nm
D)589.3 nm
E)977.7 nm
A)655.9 nm
B)546.1 nm
C)108.8 nm
D)589.3 nm
E)977.7 nm

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
31
What is the frequency of radiation which has an energy of 3.371 × 10-19 joules per photon?
A)1.697 × 1015 s-1
B)5.893 × 10-7 s-1
C)5.087 × 1014 s-1
D)1.966 × 10-15 s-1
E)6.626 × 10-34 s-1
A)1.697 × 1015 s-1
B)5.893 × 10-7 s-1
C)5.087 × 1014 s-1
D)1.966 × 10-15 s-1
E)6.626 × 10-34 s-1

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
32
What is the frequency of radiation which has an energy of 216.1 kJ per mole of photons?Hint: Pay careful attention to your units. 1 kJ = 1000 J.
A)615.9 × 1014 s-1
B)1.624 × 1014 s-1
C)1.058 × 10-10 s-1
D)5.416 × 1014 s-1
E)3.588 × 10-19 s-1
A)615.9 × 1014 s-1
B)1.624 × 1014 s-1
C)1.058 × 10-10 s-1
D)5.416 × 1014 s-1
E)3.588 × 10-19 s-1

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
33
What is the energy of one mole of photons whose wavelength is 5.461 × 102 nm?Hint: Pay careful attention to your units. 1 m = 109 nm.
A)2.191 × 10-4 J
B)2.437 × 10-12 J
C)2.191 × 105 J
D)1.376 × 106 J
E)4.06 × 10-19 J
A)2.191 × 10-4 J
B)2.437 × 10-12 J
C)2.191 × 105 J
D)1.376 × 106 J
E)4.06 × 10-19 J

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
34
Which statement is true?
A)The line spectra of elements are the same provided they belong to the same family.
B)The line spectra of elements are the same provided they belong to the same family and are combined with oxygen.
C)The line spectra of elements are the same provided they belong to the same family and are in the same physical state.
D)The line spectra of elements can be used for separation of elements from mixtures.
E)The line spectra of elements can be used to identify the elements.
A)The line spectra of elements are the same provided they belong to the same family.
B)The line spectra of elements are the same provided they belong to the same family and are combined with oxygen.
C)The line spectra of elements are the same provided they belong to the same family and are in the same physical state.
D)The line spectra of elements can be used for separation of elements from mixtures.
E)The line spectra of elements can be used to identify the elements.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
35
Which statement is true?
A)The line spectra of atoms consist of a series of white lines superimposed on a colorful background.
B)The line spectra of atoms consist of a series of white lines superimposed on a dark background.
C)The line spectra of atoms consist of a series of colorful lines superimposed on a dark background.
D)The line spectra of atoms consist of a series of dark lines superimposed on a white background.
E)The line spectra of atoms consist of a series of dark lines superimposed on a colorful background.
A)The line spectra of atoms consist of a series of white lines superimposed on a colorful background.
B)The line spectra of atoms consist of a series of white lines superimposed on a dark background.
C)The line spectra of atoms consist of a series of colorful lines superimposed on a dark background.
D)The line spectra of atoms consist of a series of dark lines superimposed on a white background.
E)The line spectra of atoms consist of a series of dark lines superimposed on a colorful background.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
36
The definite energies associated with specific wavelengths in the emission spectrum of atomic hydrogen suggest that
A)electrons have a smaller rest mass than photons.
B)photons have a smaller rest mass than electrons.
C)energy states of the electron in the hydrogen atom are quantized.
D)atomic hydrogen is more stable and has a lower potential energy than molecular hydrogen.
E)the potential energy of electrons in the atom can have any arbitrary value over a period of time, but the kinetic energy may only have certain specific values.
A)electrons have a smaller rest mass than photons.
B)photons have a smaller rest mass than electrons.
C)energy states of the electron in the hydrogen atom are quantized.
D)atomic hydrogen is more stable and has a lower potential energy than molecular hydrogen.
E)the potential energy of electrons in the atom can have any arbitrary value over a period of time, but the kinetic energy may only have certain specific values.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
37
Calculate the wavelength of the spectral line in the spectrum of hydrogen for which n1 = 1 and n2 = 3.Hint: Use the Rydberg equation to solve.
A)277 nm
B)103 nm
C)345 nm
D)397 nm
E)489 nm
A)277 nm
B)103 nm
C)345 nm
D)397 nm
E)489 nm

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
38
Calculate the wavelength of the spectral line in the spectrum of hydrogen for which n1 = 2 and n2 = 4.Hint: Use the Rydberg equation to solve.
A)207 nm
B)365 nm
C)486 nm
D)274 nm
E)131 nm
A)207 nm
B)365 nm
C)486 nm
D)274 nm
E)131 nm

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
39
Calculate the wavelength of the spectral line in the spectrum of hydrogen for which n1 = 4 and n2 = 7.Hint: Use the Rydberg equation to solve.
A)2.17 × 10-6 m
B)2.17 × 10-4 m
C)4.62 × 103 m
D)8.51 × 10-5 m
E)8.51 × 10-7 m
A)2.17 × 10-6 m
B)2.17 × 10-4 m
C)4.62 × 103 m
D)8.51 × 10-5 m
E)8.51 × 10-7 m

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
40
Which statement is true concerning the spectrum of hydrogen obtained from a gas discharge tube?
A)A photon is absorbed as the electron goes from a state with a higher energy to one with a lower energy.
B)An electron is absorbed as the electron goes from a state with a lower energy to one with a higher energy.
C)A photon is emitted as the electron goes from a state with a higher energy to one with a lower energy.
D)A photon is emitted as the electron goes from a state with a lower energy to one with a higher energy.
E)An electron is emitted as the photon goes from a state with a higher energy to one with a lower energy.
A)A photon is absorbed as the electron goes from a state with a higher energy to one with a lower energy.
B)An electron is absorbed as the electron goes from a state with a lower energy to one with a higher energy.
C)A photon is emitted as the electron goes from a state with a higher energy to one with a lower energy.
D)A photon is emitted as the electron goes from a state with a lower energy to one with a higher energy.
E)An electron is emitted as the photon goes from a state with a higher energy to one with a lower energy.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
41
Which statement is true concerning Bohr's model of the atom?
A)The model accounted for the absorption spectra of atoms but not for the emission spectra.
B)The model could account for the emission spectrum of hydrogen and for the Rydberg equation.
C)The model was based on the wave properties of the electron.
D)The model accounted for the emission spectra of atoms, but not for the absorption spectra.
E)The model was generally successful for all atoms to which it was applied.
A)The model accounted for the absorption spectra of atoms but not for the emission spectra.
B)The model could account for the emission spectrum of hydrogen and for the Rydberg equation.
C)The model was based on the wave properties of the electron.
D)The model accounted for the emission spectra of atoms, but not for the absorption spectra.
E)The model was generally successful for all atoms to which it was applied.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
42
Which statement about a hydrogen atom is false?
A)Atoms undergo the same specific energy changes.
B)When an excited atom loses energy, only a specific amount can be lost.
C)When an atom is supplied with energy, an electron drops from a higher energy level to a lower energy level.
D)When an electron drops back to a lower energy level, energy equal to the difference between the two levels is released and emitted as a photon.
E)The statements above are all true.
A)Atoms undergo the same specific energy changes.
B)When an excited atom loses energy, only a specific amount can be lost.
C)When an atom is supplied with energy, an electron drops from a higher energy level to a lower energy level.
D)When an electron drops back to a lower energy level, energy equal to the difference between the two levels is released and emitted as a photon.
E)The statements above are all true.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
43
Which statement is true of Bohr's equation, E = -b/n2?
A)E represents the energy of the proton.
B)b represents the energy of an excited electron.
C)The negative sign in the equation suggests that any electron with a finite value of n has a lower energy than an unbound electron.
D)The possible values of n are any real number
E)Each orbit is identified by its unique values for b and n.
A)E represents the energy of the proton.
B)b represents the energy of an excited electron.
C)The negative sign in the equation suggests that any electron with a finite value of n has a lower energy than an unbound electron.
D)The possible values of n are any real number
E)Each orbit is identified by its unique values for b and n.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
44
Calculate the energy required to excite a hydrogen atom by causing an electronic transition from the energy level with n = 1 to the level with n = 4. Recall that the quantized energies of the levels in the hydrogen atom are given by: 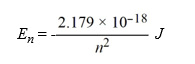
A)2.02 × 10-29 J
B)2.04 × 10-18 J
C)2.19 × 105 J
D)2.25 × 10-18 J
E)3.27 × 10-17 J
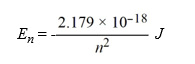
A)2.02 × 10-29 J
B)2.04 × 10-18 J
C)2.19 × 105 J
D)2.25 × 10-18 J
E)3.27 × 10-17 J

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
45
A hydrogen atom starts in the n = 1 energy level. What energy level would the atom end up in if it were to absorb 2.093 × 10-18 J of energy?Hint: Make sure to convert energy to wavelength for your calculations.
A)n = 2
B)n = 3
C)n = 4
D)n = 5
E)n = 6
A)n = 2
B)n = 3
C)n = 4
D)n = 5
E)n = 6

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
46
Calculate the wavelength, in nanometers, of light emitted by a hydrogen atom when the electron falls from an n = 7 energy level to an n = 4 energy level. Recall that the quantized energies of the levels in the hydrogen atom are given by: 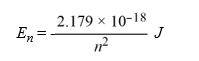
A)4.45 × 10-20 nm
B)8.51 × 102 nm
C)2.17 × 103 nm
D)1.38 × 1014 nm
E)2.16 × 103 nm
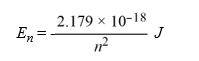
A)4.45 × 10-20 nm
B)8.51 × 102 nm
C)2.17 × 103 nm
D)1.38 × 1014 nm
E)2.16 × 103 nm

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
47
The de Broglie relationship provides a link between which two properties of the electron?
A)the mass and the charge
B)the mass and the energy
C)its energy and its charge
D)the orbit and its wavelike movements
E)its wave and particle properties
A)the mass and the charge
B)the mass and the energy
C)its energy and its charge
D)the orbit and its wavelike movements
E)its wave and particle properties

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
48
Calculate the wavelength of a particle of mass 1.00 kg traveling at 1.00 km per hour.
A)101 m
B)3.45 × 10-19 m
C)7.20 × 10-29 m
D)2.38 × 10-33 m
E)6.25 × 10--34 m
A)101 m
B)3.45 × 10-19 m
C)7.20 × 10-29 m
D)2.38 × 10-33 m
E)6.25 × 10--34 m

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
49
Calculate the wavelength of an electron (mass = 9.109 × 10-31 kg)traveling at4.38 × 106 m/s.
A)101 pm
B)166 pm
C)720 pm
D)298 pm
E)435 pm
A)101 pm
B)166 pm
C)720 pm
D)298 pm
E)435 pm

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
50
Calculate the wavelength of a hydrogen atom (mass = 1.674 × 10-27 kg)traveling at7.88 × 104 m/s.
A)3.12 × 10-2 m
B)3.12 × 10-56 m
C)2.12 × 10-32 m
D)5.02 × 10-12 m
E)1.32 × 10-22 m
A)3.12 × 10-2 m
B)3.12 × 10-56 m
C)2.12 × 10-32 m
D)5.02 × 10-12 m
E)1.32 × 10-22 m

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
51
Calculate the wavelength of a helium atom (mass = 6.65 × 10-27 kg)traveling at 1.25 km/s.
A)79.7 nm
B)79.7 pm
C)8.31 × 10-27 m
D)1.25 × 1010 m
E)831 pm
A)79.7 nm
B)79.7 pm
C)8.31 × 10-27 m
D)1.25 × 1010 m
E)831 pm

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
52
The first description of the electron in the hydrogen atom by application of the wave nature of matter was presented by
A)Louis de Broglie.
B)Werner von Heisenberg.
C)Wolfgang Pauli.
D)Ernest Rutherford.
E)Erwin Schrödinger.
A)Louis de Broglie.
B)Werner von Heisenberg.
C)Wolfgang Pauli.
D)Ernest Rutherford.
E)Erwin Schrödinger.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
53
The letter designation for the subshell in an atom is based on
A)the value of the secondary quantum number, l.
B)the value of the principal quantum number, n.
C)the value of the magnetic quantum number, ml.
D)the value of the spin quantum number, ms.
E)the transverse polarization of the optical emission from the H atom.
A)the value of the secondary quantum number, l.
B)the value of the principal quantum number, n.
C)the value of the magnetic quantum number, ml.
D)the value of the spin quantum number, ms.
E)the transverse polarization of the optical emission from the H atom.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
54
The three quantum numbers which characterize the solutions to Schrodinger's equation, describing the behavior of the electron in the H atom are usually designated as
A)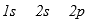
B)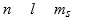
C)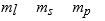
D)
E)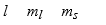
A)
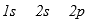
B)
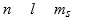
C)
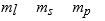
D)

E)
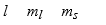

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
55
All orbitals with the same value of the principal quantum number are said to belong to the same
A)shell.
B)subshell.
C)group.
D)period.
E)class.
A)shell.
B)subshell.
C)group.
D)period.
E)class.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
56
All orbitals with the same value of the principal quantum number and the secondary quantum number are said to belong to the same
A)shell.
B)subshell.
C)group.
D)period.
E)class.
A)shell.
B)subshell.
C)group.
D)period.
E)class.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
57
The notation for the subshell with n = 5 and l = 3 is
A)5d subshell.
B)5p subshell.
C)5f subshell.
D)5g subshell.
E)5s subshell.
A)5d subshell.
B)5p subshell.
C)5f subshell.
D)5g subshell.
E)5s subshell.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
58
The notation for the subshell with n = 4 and l = 2 is
A)4d subshell.
B)4p subshell.
C)4f subshell.
D)4s subshell.
E)There is no subshell fitting this description.
A)4d subshell.
B)4p subshell.
C)4f subshell.
D)4s subshell.
E)There is no subshell fitting this description.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
59
The notation for the subshell with n = 3 and l = 3 is
A)3d subshell.
B)3f subshell.
C)3p subshell.
D)3s subshell.
E)There is no subshell fitting this description.
A)3d subshell.
B)3f subshell.
C)3p subshell.
D)3s subshell.
E)There is no subshell fitting this description.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
60
Which statement correctly summarizes allowed values of quantum numbers in atoms?
A)All of the quantum numbers are allowed to have values which are not integers.
B)Only the principal quantum number is allowed to have values which are not integers.
C)Only the spin quantum numbers are allowed to have values which are not integers.
D)Only the secondary quantum number is allowed to have values which are not integers.
E)No quantum numbers are allowed to have values which are not integers.
A)All of the quantum numbers are allowed to have values which are not integers.
B)Only the principal quantum number is allowed to have values which are not integers.
C)Only the spin quantum numbers are allowed to have values which are not integers.
D)Only the secondary quantum number is allowed to have values which are not integers.
E)No quantum numbers are allowed to have values which are not integers.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
61
The Pauli principle states that
A)an electron in an atom can move to another energy level.
B)the energy of an electron has a specific quantum number.
C)the electron trapped in an atom has particle characteristics.
D)no two electrons in the same atom can have the exact same set of quantum numbers.
E)an orbital can hold as many electrons as possible.
A)an electron in an atom can move to another energy level.
B)the energy of an electron has a specific quantum number.
C)the electron trapped in an atom has particle characteristics.
D)no two electrons in the same atom can have the exact same set of quantum numbers.
E)an orbital can hold as many electrons as possible.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
62
What is the maximum number of electrons that can fill all the orbitals of an f subshell?
A)6
B)8
C)10
D)12
E)14
A)6
B)8
C)10
D)12
E)14

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
63
What is the maximum number of electrons that can fill all the orbitals of a d subshell?
A)6
B)8
C)10
D)12
E)14
A)6
B)8
C)10
D)12
E)14

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
64
What is the maximum number of electrons that can fill all the orbitals of a p subshell?
A)2
B)3
C)4
D)6
E)10
A)2
B)3
C)4
D)6
E)10

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
65
The number of electrons required to fill all the energy levels for a shell having principal quantum number n is
A)n
B)n+1
C)2n
D)n2
E)2n2
A)n
B)n+1
C)2n
D)n2
E)2n2

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following gives a possible quantum number assignment for the last electron added to the sodium atom when developing the electron configuration using the aufbau principle? 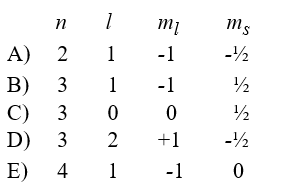


Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following gives a possible quantum number assignment for the last electron added to the oxygen atom when developing the electron configuration using the aufbau principle? 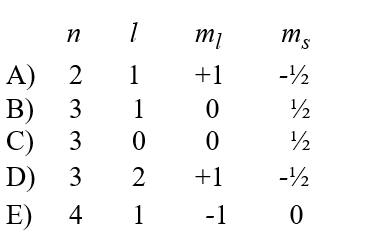


Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
68
Given the following sets of quantum numbers for n, l, ml, and ms, which one of these sets is not possible for an electron in an atom? 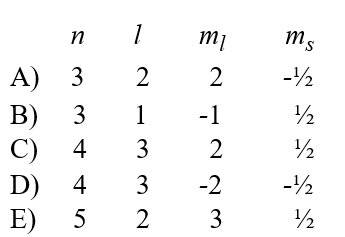


Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
69
Given the following sets of quantum numbers for n, l, ml, and ms, which one of these sets is not possible for an electron in an atom? 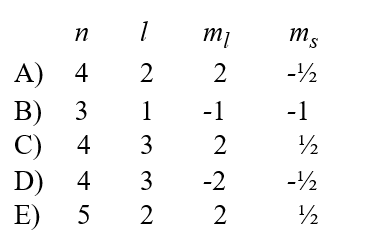


Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
70
Given the following sets of quantum numbers for n, l, ml, and ms, which one of these sets is not possible for an electron in an atom? 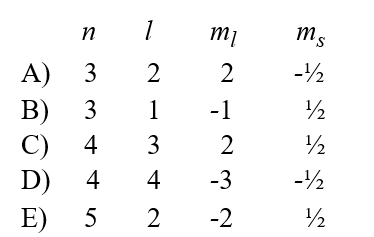


Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
71
Given the following sets of quantum numbers for n, l, ml, and ms,which one of these sets is not possible for an electron in an atom? 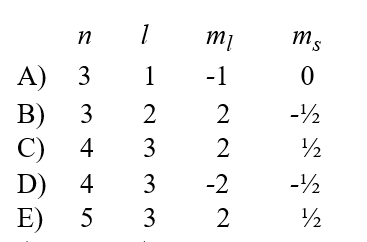


Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
72
Given the following sets of quantum numbers for n, l, ml, and ms, which one of these sets is not possible for an electron in an atom? 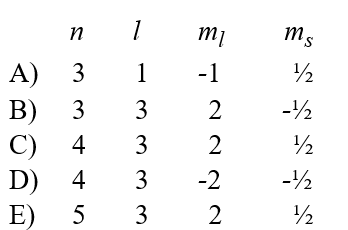


Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
73
Given the following sets of quantum numbers for n, l, ml, and ms, which one of these sets is not possible for an electron in an atom? 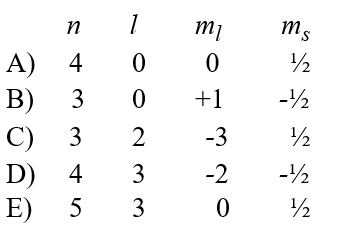
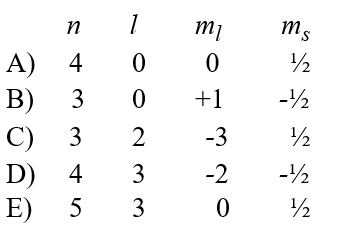

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
74
A paramagnetic element is an element
A)that always has an element in an excited state.
B)that is missing electrons.
C)that is attracted to a magnet.
D)that is not attracted to a magnet.
E)that forms a molecule with two atoms.
A)that always has an element in an excited state.
B)that is missing electrons.
C)that is attracted to a magnet.
D)that is not attracted to a magnet.
E)that forms a molecule with two atoms.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
75
A diamagnetic element is an element
A)that always has an element in an excited state.
B)that is missing electrons.
C)that is attracted to a magnet.
D)that is not attracted to a magnet.
E)that forms a molecule with two atoms.
A)that always has an element in an excited state.
B)that is missing electrons.
C)that is attracted to a magnet.
D)that is not attracted to a magnet.
E)that forms a molecule with two atoms.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
76
A possible set of quantum numbers for an electron in the partially filled subshell in a gallium atom in its ground state configuration would be 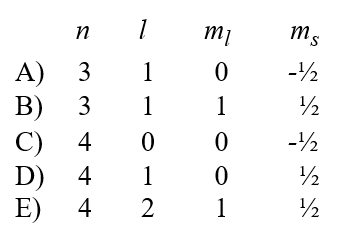
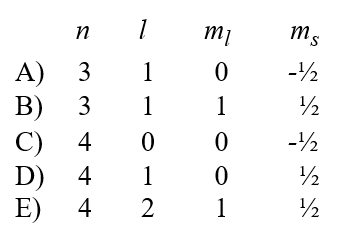

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
77
A possible set of quantum numbers for an electron in the partially filled subshell in a vanadium atom in its ground state configuration would be 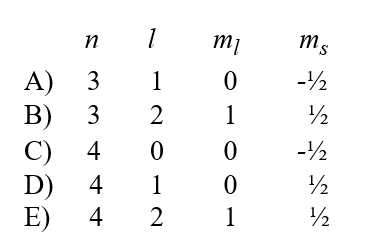


Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
78
A possible set of quantum numbers for an electron in the partially filled subshell in a potassium atom in its ground state configuration would be 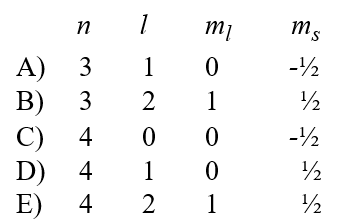


Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
79
A possible set of quantum numbers for an electron in the partially filled subshell in a germanium atom in its ground state configuration would be 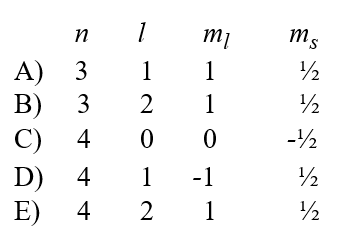


Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
80
A possible set of quantum numbers for an electron in the partially filled subshell in a technetium atom in its ground state configuration would be 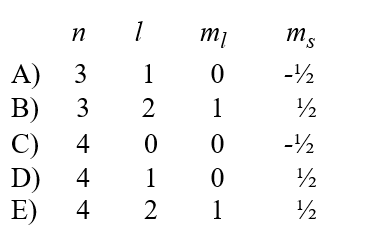


Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck


