Deck 2: Scientific Measurements
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/224
Play
Full screen (f)
Deck 2: Scientific Measurements
1
A tentative explanation used to explain observed facts or predict future results is called
A)the scientific method.
B)a scientific law.
C)a theory.
D)a hypothesis.
E)an empirical fact.
A)the scientific method.
B)a scientific law.
C)a theory.
D)a hypothesis.
E)an empirical fact.
a hypothesis.
2
A broad generalization based on the results of many experiments over time is called
A)the scientific method.
B)a scientific law.
C)a theory.
D)a hypothesis.
E)an empirical fact.
A)the scientific method.
B)a scientific law.
C)a theory.
D)a hypothesis.
E)an empirical fact.
a scientific law.
3
Which of the following is false?
A)Theories can be revised as more data becomes available.
B)A hypothesis which has successfully withstood many tests eventually can become a theory.
C)In general, a theory can be proven to be absolutely true.
D)In general, a theory cannot be proven to be absolutely true.
E)A theory is an explanation of general principles which has withstood repeated
Testing.
A)Theories can be revised as more data becomes available.
B)A hypothesis which has successfully withstood many tests eventually can become a theory.
C)In general, a theory can be proven to be absolutely true.
D)In general, a theory cannot be proven to be absolutely true.
E)A theory is an explanation of general principles which has withstood repeated
Testing.
In general, a theory can be proven to be absolutely true.
4
Which of the following gives the best description of what the scientific method is?
A)It is the process of carefully following the steps of a lab procedure.
B)It is the guidelines that are followed during laboratory measurements.
C)It is unifying principle that explains a body of facts and relations.
D)It is the process of making observations and then designing ways to evaluate or explain those observations.
E)It is a guidebook for laboratory techniques that is followed by all chemists.
A)It is the process of carefully following the steps of a lab procedure.
B)It is the guidelines that are followed during laboratory measurements.
C)It is unifying principle that explains a body of facts and relations.
D)It is the process of making observations and then designing ways to evaluate or explain those observations.
E)It is a guidebook for laboratory techniques that is followed by all chemists.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
5
An example of an element is
A)glucose, C6H12O6.
B)table salt, NaCl.
C)gold, Au.
D)an oxide of iron, Fe2O3.
E)limestone, CaCO3.
A)glucose, C6H12O6.
B)table salt, NaCl.
C)gold, Au.
D)an oxide of iron, Fe2O3.
E)limestone, CaCO3.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
6
An example of an element is
A)tin metal, Sn.
B)water, H2O.
C)benzene, C6H6.
D)carbon dioxide gas, CO2.
E)ammonium nitrate, NH4NO3.
A)tin metal, Sn.
B)water, H2O.
C)benzene, C6H6.
D)carbon dioxide gas, CO2.
E)ammonium nitrate, NH4NO3.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
7
An example of a chemical compound is
A)iron metal, Fe.
B)brass, a solution of Cu and Zn.
C)ozone gas, O3.
D)sand.
E)table salt, NaCl.
A)iron metal, Fe.
B)brass, a solution of Cu and Zn.
C)ozone gas, O3.
D)sand.
E)table salt, NaCl.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
8
An example of a chemical compound is
A)silver, Ag.
B)helium gas, He.
C)carbon dioxide gas, CO2.
D)mercury metal, Hg.
E)hydrogen gas, H2.
A)silver, Ag.
B)helium gas, He.
C)carbon dioxide gas, CO2.
D)mercury metal, Hg.
E)hydrogen gas, H2.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
9
The two types of pure substances are
A)compounds and elements.
B)compounds and solutions.
C)elements and mixtures.
D)mixtures and solutions.
E)solutions and elements.
A)compounds and elements.
B)compounds and solutions.
C)elements and mixtures.
D)mixtures and solutions.
E)solutions and elements.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
10
Which is an example of a chemical change?
A)Steam from the boiling water condenses on the ceiling.
B)The solid metal is heated until it melts.
C)The gas is cooled until it finally becomes a liquid.
D)A piece of paper burns in oxygen
E)The table salt in the warehouse container had very large chunks in it.
A)Steam from the boiling water condenses on the ceiling.
B)The solid metal is heated until it melts.
C)The gas is cooled until it finally becomes a liquid.
D)A piece of paper burns in oxygen
E)The table salt in the warehouse container had very large chunks in it.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
11
Which is an example of a physical change?
A)The milk in the box left on the table becomes sour after a few days.
B)The bit of scrap metal dissolves when placed in the container of acid.
C)The gas is cooled until it finally becomes a liquid.
D)A piece of paper burns in air with a smoky flame.
E)Bubbles are seen on the egg shell after some vinegar is poured on it.
A)The milk in the box left on the table becomes sour after a few days.
B)The bit of scrap metal dissolves when placed in the container of acid.
C)The gas is cooled until it finally becomes a liquid.
D)A piece of paper burns in air with a smoky flame.
E)Bubbles are seen on the egg shell after some vinegar is poured on it.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
12
Which is an example of a chemical change?
A)The milk in the carton became frozen after the carton was accidentally placed in the freezing compartment.
B)The bit of scrap metal was crushed by the heavy machine.
C)The gas was cooled until it eventually became a liquid.
D)The piece of paper was cut into many thin strips by the shredding machine.
E)Bubbles were seen on the egg shell after some vinegar was poured on it.
A)The milk in the carton became frozen after the carton was accidentally placed in the freezing compartment.
B)The bit of scrap metal was crushed by the heavy machine.
C)The gas was cooled until it eventually became a liquid.
D)The piece of paper was cut into many thin strips by the shredding machine.
E)Bubbles were seen on the egg shell after some vinegar was poured on it.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
13
Which is an example of a physical change?
A)Steam from the boiling water condenses on the cooler part of the ceiling.
B)The crude metal ore was first heated then combined with pure oxygen gas to make the oxide of the metal.
C)The chef made scrambled eggs for their breakfast.
D)A piece of paper burns in air with a smoky flame.
E)The table salt in the warehouse was used to make some of the polymeric material.
A)Steam from the boiling water condenses on the cooler part of the ceiling.
B)The crude metal ore was first heated then combined with pure oxygen gas to make the oxide of the metal.
C)The chef made scrambled eggs for their breakfast.
D)A piece of paper burns in air with a smoky flame.
E)The table salt in the warehouse was used to make some of the polymeric material.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
14
Which is an example of a physical change?
A)Water freezing at 0°C.
B)Burning gasoline.
C)A potato turns brown after being cut open and left out.
D)Heating magnesium metal causes it to turn into an off white solid.
E)When exposed to certain metals, hydrogen peroxide will bubble and fizz.
A)Water freezing at 0°C.
B)Burning gasoline.
C)A potato turns brown after being cut open and left out.
D)Heating magnesium metal causes it to turn into an off white solid.
E)When exposed to certain metals, hydrogen peroxide will bubble and fizz.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
15
Which is an example of both a physical and a chemical change? Hint: Think about the what is occurring over the course of time in each choice. Remember, phase change is physical.
A)The milk in the carton became frozen after the carton was accidentally placed in the freezing compartment overnight.
B)The bit of scrap metal was removed to the junkyard after being crushed by the heavy machine.
C)The gas was collected in a flask and cooled until it eventually became a liquid.
D)The old parchment became dry after being placed in the hot oven, but then was charred since it was not removed in the specified time.
E)Bubbles were seen on the egg shell when the vinegar was poured on it.
A)The milk in the carton became frozen after the carton was accidentally placed in the freezing compartment overnight.
B)The bit of scrap metal was removed to the junkyard after being crushed by the heavy machine.
C)The gas was collected in a flask and cooled until it eventually became a liquid.
D)The old parchment became dry after being placed in the hot oven, but then was charred since it was not removed in the specified time.
E)Bubbles were seen on the egg shell when the vinegar was poured on it.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
16
Which is an example of both a physical and a chemical change? Hint: Think about where the wax could have gone and how.
A)The milk in the carton became frozen because the carton was accidentally placed in the freezing compartment overnight.
B)The bit of scrap metal was removed to the junkyard after being crushed by the heavy machine.
C)The gas was collected in a flask and cooled until it eventually became a liquid.
D)The old parchment became dry when it was placed in the warm oven for ten minutes.
E)As a candle burns the wax melts down the side. When the candle is done burning, much of the wax is gone.
A)The milk in the carton became frozen because the carton was accidentally placed in the freezing compartment overnight.
B)The bit of scrap metal was removed to the junkyard after being crushed by the heavy machine.
C)The gas was collected in a flask and cooled until it eventually became a liquid.
D)The old parchment became dry when it was placed in the warm oven for ten minutes.
E)As a candle burns the wax melts down the side. When the candle is done burning, much of the wax is gone.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
17
An example of a chemical change is
A)the mixing of glucose with table salt.
B)the dissolving of table salt in water.
C)sodium combining with chlorine to form table salt.
D)mixing rust with sand.
E)mixing chalk with helium in a balloon.
A)the mixing of glucose with table salt.
B)the dissolving of table salt in water.
C)sodium combining with chlorine to form table salt.
D)mixing rust with sand.
E)mixing chalk with helium in a balloon.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the examples below is a chemical change?
A)A bottle of wine completely evaporates in two weeks.
B)Bleach changes the color of the stain on the white shirt.
C)The 'dry ice' (solid CO2)changes to vapor.
D)Bubbles form in the water when He gas is blown into the water.
E)These are all examples of chemical change.
A)A bottle of wine completely evaporates in two weeks.
B)Bleach changes the color of the stain on the white shirt.
C)The 'dry ice' (solid CO2)changes to vapor.
D)Bubbles form in the water when He gas is blown into the water.
E)These are all examples of chemical change.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
19
Which is an example of a chemical change?
A)the separation of air into oxygen, nitrogen, and other components
B)the separation of a compound into its elements
C)the separation of gases from liquids
D)the separation of a mixture into its components
E)the separation of solids from liquids
A)the separation of air into oxygen, nitrogen, and other components
B)the separation of a compound into its elements
C)the separation of gases from liquids
D)the separation of a mixture into its components
E)the separation of solids from liquids

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
20
Which one of the following is a physical change?
A)When ignited with a match in open air, paper burns.
B)In cold weather, water condenses on the inside surface of single pane windows.
C)When treated with bleach, some dyed fabrics change color.
D)When heated for a period of time, sugar turns dark brown.
E)Grape juice left in an open, unrefrigerated container turns sour.
A)When ignited with a match in open air, paper burns.
B)In cold weather, water condenses on the inside surface of single pane windows.
C)When treated with bleach, some dyed fabrics change color.
D)When heated for a period of time, sugar turns dark brown.
E)Grape juice left in an open, unrefrigerated container turns sour.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
21
Which is an example of a physical change?
A)A piece of 'dry ice' (solid CO2)changes to vapor.
B)A bottle of wine turns into vinegar in a few months.
C)Bleach changes the color of a stain on a white shirt.
D)Bubbles form on an egg shell when it is placed in vinegar.
E)A portion of a figurine dissolves after being placed in the container of acid.
A)A piece of 'dry ice' (solid CO2)changes to vapor.
B)A bottle of wine turns into vinegar in a few months.
C)Bleach changes the color of a stain on a white shirt.
D)Bubbles form on an egg shell when it is placed in vinegar.
E)A portion of a figurine dissolves after being placed in the container of acid.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
22
Which one of the following examples does not involve a chemical change?
A)A fish that is left for some time in an unrefrigerated place decomposes.
B)Apple juice that is left in an open bottle ferments.
C)A loaf of bread rises and its volume expands when it is baked in an oven.
D)When a lake starts to freeze in winter, ice is formed on the surface.
E)When sugar is fermented under certain conditions, alcohol is produced.
A)A fish that is left for some time in an unrefrigerated place decomposes.
B)Apple juice that is left in an open bottle ferments.
C)A loaf of bread rises and its volume expands when it is baked in an oven.
D)When a lake starts to freeze in winter, ice is formed on the surface.
E)When sugar is fermented under certain conditions, alcohol is produced.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
23
Which one of the following is a chemical change?
A)When blood is mixed with 3% hydrogen peroxide solution, it changes color.
B)When water is boiled, it forms steam.
C)When a solid stick of butter is heated, it becomes a liquid.
D)When blue paint is mixed with yellow paint, a green colored paint is obtained.
E)When a bar of gold metal is pounded with a hammer, it flattens out.
A)When blood is mixed with 3% hydrogen peroxide solution, it changes color.
B)When water is boiled, it forms steam.
C)When a solid stick of butter is heated, it becomes a liquid.
D)When blue paint is mixed with yellow paint, a green colored paint is obtained.
E)When a bar of gold metal is pounded with a hammer, it flattens out.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is not a chemical change?
A)A nail rusts when exposed to air and moisture.
B)Milk turns sour if left unrefrigerated.
C)Yeast produces carbon dioxide to help bread rise.
D)Copper is molded with heat to form pipes.
E)Mixing baking soda and vinegar causes fizzing and bubbling.
A)A nail rusts when exposed to air and moisture.
B)Milk turns sour if left unrefrigerated.
C)Yeast produces carbon dioxide to help bread rise.
D)Copper is molded with heat to form pipes.
E)Mixing baking soda and vinegar causes fizzing and bubbling.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
25
Each of the following properties of a sample of a pure substance can be used for identification except its
A)density.
B)freezing point temperature.
C)mass.
D)melting point temperature.
E)solubility in water.
A)density.
B)freezing point temperature.
C)mass.
D)melting point temperature.
E)solubility in water.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
26
A physical property is one that
A)can be observed without changing the chemical identity of a substance.
B)is based on one particular scientific law.
C)describes a chemical reaction that a substance undergoes.
D)cannot be seen with the naked eye.
E)is considered hypothetical in origin.
A)can be observed without changing the chemical identity of a substance.
B)is based on one particular scientific law.
C)describes a chemical reaction that a substance undergoes.
D)cannot be seen with the naked eye.
E)is considered hypothetical in origin.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
27
All of these statements describe properties of sodium. Which one describes a physical property of sodium?
A)Sodium's surface turns black when first exposed to air.
B)Sodium is a solid at 25°C and changes to a liquid when heated to 98°C.
C)When exposed to water, sodium reacts violently and a gas is formed.
D)When placed in contact with chlorine, sodium forms a compound that melts at 801°C.
E)If solid sodium is put in ethanol, it will produce hydrogen gas.
A)Sodium's surface turns black when first exposed to air.
B)Sodium is a solid at 25°C and changes to a liquid when heated to 98°C.
C)When exposed to water, sodium reacts violently and a gas is formed.
D)When placed in contact with chlorine, sodium forms a compound that melts at 801°C.
E)If solid sodium is put in ethanol, it will produce hydrogen gas.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
28
Water can also exist as a gas that is called
A)ice.
B)steam.
C)evaporation.
D)molecules.
E)atomic water.
A)ice.
B)steam.
C)evaporation.
D)molecules.
E)atomic water.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following describes a chemical property?
A)A property based solely on the scientific method.
B)A property which is based on a particular scientific law.
C)A property which describes a change in composition that a substance undergoes.
D)A property which cannot be seen.
E)A property which is considered hypothetical.
A)A property based solely on the scientific method.
B)A property which is based on a particular scientific law.
C)A property which describes a change in composition that a substance undergoes.
D)A property which cannot be seen.
E)A property which is considered hypothetical.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
30
All of these statements describe properties of tin. Which one describes a chemical property of tin?
A)Tin can be hammered into a thin sheet.
B)The density of white tin is 7.365 g cm-3.
C)Tin melts at 231.9°C.
D)When a bar of tin is bent, it emits an audible "cry."
E)Tin dissolves slowly in cold, dilute hydrochloric acid, but it dissolves readily in concentrated hydrochloric acid.
A)Tin can be hammered into a thin sheet.
B)The density of white tin is 7.365 g cm-3.
C)Tin melts at 231.9°C.
D)When a bar of tin is bent, it emits an audible "cry."
E)Tin dissolves slowly in cold, dilute hydrochloric acid, but it dissolves readily in concentrated hydrochloric acid.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
31
Which is an example of a chemical property?
A)combustibility
B)volatility
C)viscosity
D)malleability
E)ductility
A)combustibility
B)volatility
C)viscosity
D)malleability
E)ductility

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
32
Which is an example of an intensive property of matter?
A)color
B)volume
C)mass
D)weight
E)length
A)color
B)volume
C)mass
D)weight
E)length

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
33
Which is an example of an intensive property of matter?
A)height
B)volume
C)length
D)weight
E)melting point
A)height
B)volume
C)length
D)weight
E)melting point

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
34
Which is an example of an extensive property of matter?
A)color
B)density
C)mass
D)melting point
E)flash point
A)color
B)density
C)mass
D)melting point
E)flash point

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
35
Which is an example of an extensive property of matter?
A)surface area
B)boiling point
C)density
D)hardness
E)freezing point
A)surface area
B)boiling point
C)density
D)hardness
E)freezing point

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
36
The kilo is
A)a unit of mass.
B)a unit used in medical terminology.
C)a decimal multiplier in the metric system.
D)a unit of speed.
E)a volume unit used by the DEA (drug enforcement agency).
A)a unit of mass.
B)a unit used in medical terminology.
C)a decimal multiplier in the metric system.
D)a unit of speed.
E)a volume unit used by the DEA (drug enforcement agency).

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
37
The SI base units of temperature and mass, respectively, are
A)degree and gram.
B)kelvin and kilogram.
C)degree Celsius and milligram.
D)degree and kilogram.
E)kelvin and gram.
A)degree and gram.
B)kelvin and kilogram.
C)degree Celsius and milligram.
D)degree and kilogram.
E)kelvin and gram.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
38
The SI base units of length and mass, respectively, are
A)centimeter and gram.
B)inch and kilogram.
C)meter and kilogram.
D)meter and gram.
E)inch and pound.
A)centimeter and gram.
B)inch and kilogram.
C)meter and kilogram.
D)meter and gram.
E)inch and pound.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is an SI base unit?
A)degree Fahrenheit
B)foot
C)milliliter
D)ampere
E)gram
A)degree Fahrenheit
B)foot
C)milliliter
D)ampere
E)gram

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
40
Which is a unit of surface area of a spherical object?
A)pascal
B)joule
C)square meter
D)cubic centimeter
E)kilometer
A)pascal
B)joule
C)square meter
D)cubic centimeter
E)kilometer

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
41
The SI derived unit for area is
A)square centimeter.
B)square yard.
C)square kilometer.
D)square meter.
E)pascal.
A)square centimeter.
B)square yard.
C)square kilometer.
D)square meter.
E)pascal.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
42
The density of an object is the ratio of its mass to its volume. What is the derived SI unit for density?
A)kg m/s3
B)kg m/s
C)kg/m3
D)m/s2
E)pounds per cubic inches
A)kg m/s3
B)kg m/s
C)kg/m3
D)m/s2
E)pounds per cubic inches

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
43
The momentum of an object is its mass times its velocity. What is the derived SI unit for momentum?
A)kg/m
B)kg m/s
C)g m/s
D)m/s2
E)pounds per inch
A)kg/m
B)kg m/s
C)g m/s
D)m/s2
E)pounds per inch

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
44
The force experienced by an object is its mass times its acceleration. What is the derived SI unit for force?
A)kg m/s2
B)cg m/s
C)g m/s2
D)m/s2
E)pounds per inches squared
A)kg m/s2
B)cg m/s
C)g m/s2
D)m/s2
E)pounds per inches squared

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
45
The volume of a cylinder is ? r2h, where r is the radius and h is the height. What is the derived SI unit for the volume of a cylinder? Hint: The SI unit for length is the meter, m.
A)kg m/s3
B)liter
C)cm3
D)m3
E)cubic inches
A)kg m/s3
B)liter
C)cm3
D)m3
E)cubic inches

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
46
The SI prefixes giga and micro, indicate respectively:
A)109 and 10-6
B)10-9 and 10-6
C)106 and 10-3
D)103 and 10-3
E)10-9 and 10-3
A)109 and 10-6
B)10-9 and 10-6
C)106 and 10-3
D)103 and 10-3
E)10-9 and 10-3

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
47
The SI prefixes mega and nano indicate, respectively:
A)109 and 10-6
B)10-6 and 109
C)106 and 10-9
D)106 and 109
E)10-6 and 10-9
A)109 and 10-6
B)10-6 and 109
C)106 and 10-9
D)106 and 109
E)10-6 and 10-9

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
48
The SI prefixes kilo and centi indicate, respectively:
A)103 and 10-2
B)106 and 10-1
C)10-3 and 10-2
D)10-6 and 102
E)102 and 10-3
A)103 and 10-2
B)106 and 10-1
C)10-3 and 10-2
D)10-6 and 102
E)102 and 10-3

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
49
Which is the longest measurement?
A)10 mm
B)10 dm
C)10 cm
D)10 ?m
E)They are all the same measurement.
A)10 mm
B)10 dm
C)10 cm
D)10 ?m
E)They are all the same measurement.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
50
Which is the shortest measurement?
A)10 mm
B)1 km
C)10 cm
D)10 ?m
E)They are all the same measurement.
A)10 mm
B)1 km
C)10 cm
D)10 ?m
E)They are all the same measurement.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
51
Which is the shortest measurement?
A)10 mm
B)1 km
C)10 cm
D)10 in
E)They are all the same measurement.
A)10 mm
B)1 km
C)10 cm
D)10 in
E)They are all the same measurement.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
52
Which is the longest measurement?
A)10 mm
B)10 dm
C)10 cm
D)10 in
E)They are all the same measurement.
A)10 mm
B)10 dm
C)10 cm
D)10 in
E)They are all the same measurement.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
53
What is the number needed to complete the following: 1 dm = ________ m? Hint: Think about the prefix deci (d)and what it means.
A)10
B)20
C)1
D)0.1
E)0.01
A)10
B)20
C)1
D)0.1
E)0.01

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
54
What is the number needed to complete the following: 1 m = ________ pm?
A)10-6
B)2.0 × 10-9
C)10-12
D)0.1
E)1012
A)10-6
B)2.0 × 10-9
C)10-12
D)0.1
E)1012

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
55
What is the number needed to complete the following: 1 g = ________ kg?
A)103
B)2.0 × 10-9
C)10-3
D)0.1
E)1012
A)103
B)2.0 × 10-9
C)10-3
D)0.1
E)1012

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
56
What is the number needed to complete the following: 1 g = ________ g?
A)106
B)10-9
C)10-3
D)0.1
E)10-2
A)106
B)10-9
C)10-3
D)0.1
E)10-2

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
57
The boiling point of chlorine is 34.6 °C. This temperature expressed in Kelvin is
A)30.3 K
B)177.4 K
C)238.6 K
D)243.0 K
E)307.6 K
A)30.3 K
B)177.4 K
C)238.6 K
D)243.0 K
E)307.6 K

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
58
Convert 25.4 K to °C.
A)30.3 °C
B)-247.8 °C
C)-38.6 °C
D)-13.8 °C
E)-107.6 °C
A)30.3 °C
B)-247.8 °C
C)-38.6 °C
D)-13.8 °C
E)-107.6 °C

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
59
Convert 79.0°F to °C.
A)79.0 °C
B)26.1 °C
C)352 °C
D)45 °C
E)111 °C
A)79.0 °C
B)26.1 °C
C)352 °C
D)45 °C
E)111 °C

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
60
Ammonia boils at -33.4°C. What temperature is this in °F?
A)-60.1 °F
B)-92.1 °F
C)-28.1 °F
D)-18.5 °F
E)13.5 °F
A)-60.1 °F
B)-92.1 °F
C)-28.1 °F
D)-18.5 °F
E)13.5 °F

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
61
The boiling point of barium is 725 °C. Determine the equivalent value in °F.
A)435 °F
B)1337 °F
C)1247 °F
D)1392 °F
E)1273 °F
A)435 °F
B)1337 °F
C)1247 °F
D)1392 °F
E)1273 °F

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
62
The melting point of a metal is listed in one handbook as 630.5 °C. Determine the equivalent value in °F.
A)382.41 °F
B)1103.3 °F
C)1077.7 °F
D)1166.9 °F
E)1192.9 °F
A)382.41 °F
B)1103.3 °F
C)1077.7 °F
D)1166.9 °F
E)1192.9 °F

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
63
The melting point of lead acetate, a white solid, is 280 °C. Determine the melting point of this compound in units of °F.
A)446 °F
B)472 °F
C)504 °F
D)536 °F
E)562 °F
A)446 °F
B)472 °F
C)504 °F
D)536 °F
E)562 °F

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
64
Convert 60.0°F to Kelvin.
A)289 K
B)15.6 K
C)140 K
D)413 K
E)333 K
A)289 K
B)15.6 K
C)140 K
D)413 K
E)333 K

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
65
On a day in the summer of 1992, the temperature fell from 98 °F to 75 °F in just three hours. The temperature drop expressed in Celsius degrees (°C)was Hint: You must convert the temperatures before taking the difference.
A)13 °C
B)41 °C
C)45 °C
D)9 °C
E)75 °C
A)13 °C
B)41 °C
C)45 °C
D)9 °C
E)75 °C

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
66
On a day in the summer of 1976, the temperature fell from 95 °F to 75 °F in just three hours. The temperature drop expressed in Celsius degrees (°C)was Hint: You must convert the temperatures before taking the difference.
A)11 °C.
B)13 °C.
C)18 °C.
D)20 °C.
E)-12 °C.
A)11 °C.
B)13 °C.
C)18 °C.
D)20 °C.
E)-12 °C.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
67
The melting point of antimony is listed in one handbook as 1167.3 °F. Expressed in Kelvin this temperature would be
A)357.6 K.
B)496.8 K.
C)583.7 K.
D)894.2 K.
E)903.9 K.
A)357.6 K.
B)496.8 K.
C)583.7 K.
D)894.2 K.
E)903.9 K.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
68
The highest temperature recorded in the athletic field house when the cooling units were being replaced and upgraded was 122.0 °F. Express this temperature in Kelvin.
A)323.2 K
B)337.6 K
C)341.0 K
D)435.2 K
E)492.8 K
A)323.2 K
B)337.6 K
C)341.0 K
D)435.2 K
E)492.8 K

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
69
A metal alloy melts at 874 K. What is this temperature in °F?
A)302 °F
B)365 °F
C)1050 °F
D)1082 °F
E)1114 °F
A)302 °F
B)365 °F
C)1050 °F
D)1082 °F
E)1114 °F

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
70
The boiling point of carbonyl selenide is 251.4 K. What is this temperature in °F?
A)-7.2 °F
B)44.1 °F
C)96.7 °F
D)0.00 °F
E)+18.5 °F
A)-7.2 °F
B)44.1 °F
C)96.7 °F
D)0.00 °F
E)+18.5 °F

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
71
A number resulting from a measurement was properly expressed in scientific notation as 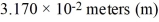 . The number could also be written correctly as
. The number could also be written correctly as
A)0.0317 m
B)0.03170 m
C)0.032 m
D)317 m
E)317.0 m
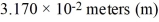 . The number could also be written correctly as
. The number could also be written correctly asA)0.0317 m
B)0.03170 m
C)0.032 m
D)317 m
E)317.0 m

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
72
How many significant figures does the number 1.030 × 107 have?
A)2
B)3
C)4
D)7
E)1
A)2
B)3
C)4
D)7
E)1

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
73
How many significant figures are in 5100.0 L?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
74
An electronic balance used in the mailroom displays tenths of a kilogram from 0 to 140 kg. How many significant figures should be used to express the mass of a package which has a mass between 80.2 and 83.5 kg?
A)3
B)5
C)4
D)2
E)1
A)3
B)5
C)4
D)2
E)1

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
75
Which response gives the correct number of significant figures for each of the three following measurements, in order? 7.103 cm, 0.00005 inch, and 1.3400 × 104 dm3
A)3, 5, and 4
B)3, 1, and 3
C)4, 1, and 3
D)4, 1, and 5
E)4, 5, and 5
A)3, 5, and 4
B)3, 1, and 3
C)4, 1, and 3
D)4, 1, and 5
E)4, 5, and 5

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
76
How many significant figures should be displayed in the result of the operation, 8.5201 + 1.93?
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
77
The number 0.0030600 is properly expressed in scientific notation as
A)3.0600 × 102.
B)0.30600 × 102.
C)0.306 × 102.
D)3.06 × 103.
E)3.0600 × 103.
A)3.0600 × 102.
B)0.30600 × 102.
C)0.306 × 102.
D)3.06 × 103.
E)3.0600 × 103.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
78
The number 0.02100 is properly expressed in scientific notation as
A)0.21 × 101.
B)2.1 × 102.
C)2.100 × 102.
D)21.0 × 103.
E)2.10 × 102.
A)0.21 × 101.
B)2.1 × 102.
C)2.100 × 102.
D)21.0 × 103.
E)2.10 × 102.

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
79
After evaluating the expression, 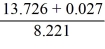 how many significant figures should be displayed in the result?
how many significant figures should be displayed in the result?
A)1
B)2
C)3
D)4
E)5
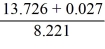 how many significant figures should be displayed in the result?
how many significant figures should be displayed in the result?A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck
80
Give the correct answer for the following problem with the correct number of significant figures.(13.7 + 0.027)÷ 8.221
A)1.7
B)1.67
C)1.670
D)1.703
E)1.699
A)1.7
B)1.67
C)1.670
D)1.703
E)1.699

Unlock Deck
Unlock for access to all 224 flashcards in this deck.
Unlock Deck
k this deck


