Deck 20: The Main Group Elements
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/29
Play
Full screen (f)
Deck 20: The Main Group Elements
1
In the reaction of trimethyl amine with trimethyl boron,
A) the product is [N(CH3)4+][B(CH3)2-].
B) trimethyl boron is the Lewis base.
C) the product is (CH3)3N-B(CH3)3.
D) trimethyl amine is the Lewis acid.
E) no reaction occurs.
A) the product is [N(CH3)4+][B(CH3)2-].
B) trimethyl boron is the Lewis base.
C) the product is (CH3)3N-B(CH3)3.
D) trimethyl amine is the Lewis acid.
E) no reaction occurs.
the product is (CH3)3N-B(CH3)3.
2
Which of the following are Lewis acids? 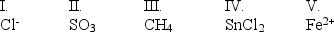
A) I, II, and III
B) II, III, and IV
C) II, IV, and V
D) II and III
E) IV and V
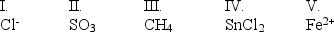
A) I, II, and III
B) II, III, and IV
C) II, IV, and V
D) II and III
E) IV and V
II, IV, and V
3
Which of the following are Lewis bases? 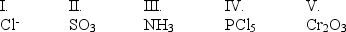
A) I, II, and III
B) I, III, and V
C) II, IV, and V
D) I and III
E) II and IV
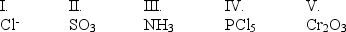
A) I, II, and III
B) I, III, and V
C) II, IV, and V
D) I and III
E) II and IV
I, III, and V
4
What reactants likely lead to formation of the Lewis acid-base adduct shown below? 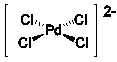
A) Pd2+ and Cl2
B) Pd and Cl-
C) Pd and SnCl4
D) Pd2+ and Cl-
E) PdCl2 and Sn
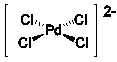
A) Pd2+ and Cl2
B) Pd and Cl-
C) Pd and SnCl4
D) Pd2+ and Cl-
E) PdCl2 and Sn

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
5
Which statements are true about the following reaction?
PF5 + HF H+ + PF6-
I. HF is a Brønsted acid and a Lewis acid.
II. PF5 is the Lewis acid.
III. The F- ion is the Lewis base.
IV. The orbital used in bonding is a dsp3 hybrid orbital.
V. PF6-is a Lewis acid.
A) I and V
B) II and III
C) I, III and IV
D) III and V
E) I and III
PF5 + HF H+ + PF6-
I. HF is a Brønsted acid and a Lewis acid.
II. PF5 is the Lewis acid.
III. The F- ion is the Lewis base.
IV. The orbital used in bonding is a dsp3 hybrid orbital.
V. PF6-is a Lewis acid.
A) I and V
B) II and III
C) I, III and IV
D) III and V
E) I and III

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
6
Identify the Lewis acid and base in the reaction of trimethyl amine with trimethyl boron.
A) Base: trimethyl amine; acid trimethyl boron
B) Base: trimethyl boron; acid trimethyl amine
C) Neither acts as an acid nor a base.
A) Base: trimethyl amine; acid trimethyl boron
B) Base: trimethyl boron; acid trimethyl amine
C) Neither acts as an acid nor a base.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following groups of Lewis acids is in order of increasing hardness?
A) Cd2+, Zn2+, Ag+
B) Ag+, Zn2+ Cd2+
C) Ag+, Cd2+, Zn2+
D) Cd2+, Ag+, Zn2+
E) Zn2+, Ag+, Cd2+
A) Cd2+, Zn2+, Ag+
B) Ag+, Zn2+ Cd2+
C) Ag+, Cd2+, Zn2+
D) Cd2+, Ag+, Zn2+
E) Zn2+, Ag+, Cd2+

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following groups of Lewis bases is in order of decreasing hardness?
A) N(CH3)3, NH3, P(CH3)3
B) NH3, P(CH3)3, N(CH3)3
C) P(CH3)3, N(CH3)3, NH3
D) NH3, N(CH3)3, P(CH3)3
E) N(CH3)3, P(CH3)3, NH3
A) N(CH3)3, NH3, P(CH3)3
B) NH3, P(CH3)3, N(CH3)3
C) P(CH3)3, N(CH3)3, NH3
D) NH3, N(CH3)3, P(CH3)3
E) N(CH3)3, P(CH3)3, NH3

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
9
The reactions of Group 13 chlorides (BCl3, AlCl3, GaCl3, InCl3) with bases are predicted well by the HSAB principle. Which of the following is the predicted order of reactivity (completeness of adduct formation) of these compounds toward P(CH2CH3)3?
A) AlCl3 < InCl3 < BCl3 < GaCl3
B) BCl3< GaCl3 < AlCl3 < InCl3
C) BCl3 < AlCl3 < GaCl3 < InCl3
D) AlCl3 < BCl3 < GaCl3 < InCl3
E) InCl3 < GaCl3 < AlCl3 < BCl3
A) AlCl3 < InCl3 < BCl3 < GaCl3
B) BCl3< GaCl3 < AlCl3 < InCl3
C) BCl3 < AlCl3 < GaCl3 < InCl3
D) AlCl3 < BCl3 < GaCl3 < InCl3
E) InCl3 < GaCl3 < AlCl3 < BCl3

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
10
Identify the following as Lewis acids or bases and rank from hardest to softest: Al3+, Tl3+, Tl+.
A) bases Al3+, Tl3+, Tl+
B) acids Al3+, Tl3+, Tl+
C) acids Tl+, Tl3+, Al3+
D) bases Tl+, Tl3+, Al3+
E) acids Al3+, Tl+, Tl3+
A) bases Al3+, Tl3+, Tl+
B) acids Al3+, Tl3+, Tl+
C) acids Tl+, Tl3+, Al3+
D) bases Tl+, Tl3+, Al3+
E) acids Al3+, Tl+, Tl3+

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
11
Identify the following as Lewis acids or bases and rank from hardest to softest: SbH3, PH3, PF3.
A) bases SbH3, PH3, PF3
B) acids PF3, PH3, SbH3
C) acids SbH3, PH3, PF3
D) bases PF3, PH3, SbH3
E) acids PH3, PF3, SbH3
A) bases SbH3, PH3, PF3
B) acids PF3, PH3, SbH3
C) acids SbH3, PH3, PF3
D) bases PF3, PH3, SbH3
E) acids PH3, PF3, SbH3

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
12
The substances below are main group compounds that follow the hard-soft acid-base principles quite reliably:
I. Al(CH3)3
II. MgCl2
III. AlCl3
IV. LiCl
V. LiCH3
VI. Mg(CH3)2
Referring to these compounds, which of the (unbalanced) equations below is expected to proceed?
A) Al(CH3)3 + MgCl2 AlCl3 + Mg(CH3)2
B) LiCl + Al(CH3)3 LiCH3 + AlCl3
C) LiCH3 + MgCl2 LiCl + Mg(CH3)2
D) Mg(CH3)2 + LiCl LiCH3 + MgCl2
I. Al(CH3)3
II. MgCl2
III. AlCl3
IV. LiCl
V. LiCH3
VI. Mg(CH3)2
Referring to these compounds, which of the (unbalanced) equations below is expected to proceed?
A) Al(CH3)3 + MgCl2 AlCl3 + Mg(CH3)2
B) LiCl + Al(CH3)3 LiCH3 + AlCl3
C) LiCH3 + MgCl2 LiCl + Mg(CH3)2
D) Mg(CH3)2 + LiCl LiCH3 + MgCl2

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
13
Potassium is found in nature most often as a chloride salt, whereas copper and mercury more often occur as the sulphides. The best explanation for this observation is
A) potassium is softer than copper or mercury.
B) the coinage metals are smaller than potassium.
C) the coinage metals prefer the +2 oxidation state.
D) potassium is less electronegative than copper.
E) the coinage metal ions are softer than potassium.
A) potassium is softer than copper or mercury.
B) the coinage metals are smaller than potassium.
C) the coinage metals prefer the +2 oxidation state.
D) potassium is less electronegative than copper.
E) the coinage metal ions are softer than potassium.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
14
Are the chlorides of Pb, Pb(II) chloride and Pb(IV) chloride solids, liquids, or gases at room temperature?
A) PbCl2: solid; PbCl4: solid
B) PbCl2: liquid; PbCl4: liquid
C) PbCl2: solid; PbCl4: liquid
D) PbCl2: liquid; PbCl4: solid
E) PbCl2: gas; PbCl4: liquid
A) PbCl2: solid; PbCl4: solid
B) PbCl2: liquid; PbCl4: liquid
C) PbCl2: solid; PbCl4: liquid
D) PbCl2: liquid; PbCl4: solid
E) PbCl2: gas; PbCl4: liquid

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
15
What reasons are important for the use of Na3AlF6 in the production of aluminum?
I. The use of Na3AlF6 allows for electrolysis at lower temperature.
II. The fluoride will react with the electrodes.
III. The oxide ions combine with the fluoride ions.
IV. The fluoride is not readily oxidized.
V. The aluminum is more easily oxidized when bound to fluoride.
A) II and III
B) IV and V
C) I and V
D) I and IV
E) I, III, and V
I. The use of Na3AlF6 allows for electrolysis at lower temperature.
II. The fluoride will react with the electrodes.
III. The oxide ions combine with the fluoride ions.
IV. The fluoride is not readily oxidized.
V. The aluminum is more easily oxidized when bound to fluoride.
A) II and III
B) IV and V
C) I and V
D) I and IV
E) I, III, and V

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
16
Why are heavy metals such as lead(II) and cadmium(II) toxic?
A) They can replace oxygen atoms in proteins.
B) They can bind to sulphur atoms on proteins.
C) They are hard Lewis acids.
D) They are insoluble in aqueous solution and will precipitate.
E) They form ionic compounds.
A) They can replace oxygen atoms in proteins.
B) They can bind to sulphur atoms on proteins.
C) They are hard Lewis acids.
D) They are insoluble in aqueous solution and will precipitate.
E) They form ionic compounds.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following ionic species result when boric acid, H3BO3, is added to water:H3O+, H2BO3-, HBO32-, BO33-, B(OH)4-, OH-?
A) H3O+, H2BO3-,
B) H3O+, H2BO3-, HBO32-
C) H3O+, H2BO3-, HBO32-, BO33
D) H3O+, B(OH)4-
E) H3O+, OH-
A) H3O+, H2BO3-,
B) H3O+, H2BO3-, HBO32-
C) H3O+, H2BO3-, HBO32-, BO33
D) H3O+, B(OH)4-
E) H3O+, OH-

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following boron halides has the most -bonding?
A) BI3
B) BBr3
C) BCl3
D) BF3
E) BF4-
A) BI3
B) BBr3
C) BCl3
D) BF3
E) BF4-

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
19
What is the product of the reaction of silicon-copper alloy with chlorobutane?
A)
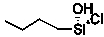
B)

C)
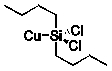
D)
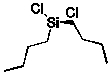
E) Cu metal
A)

B)

C)

D)

E) Cu metal

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
20
Why is SiO2 a solid and CO2 a gas?
A) A Si atom is heavier than a C carbon.
B) Si has more electrons than C.
C) Intermolecular forces between SiO2 molecules are stronger than those between CO2 molecules.
D) It is preferable to form 4 Si - O single bonds rather than 2 Si - O double bonds.
E) SiO2 forms ionic bonds.
A) A Si atom is heavier than a C carbon.
B) Si has more electrons than C.
C) Intermolecular forces between SiO2 molecules are stronger than those between CO2 molecules.
D) It is preferable to form 4 Si - O single bonds rather than 2 Si - O double bonds.
E) SiO2 forms ionic bonds.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
21
Silicon tetrachloride has Lewis acidic characteristics, whereas carbon tetrachloride does not. What orbital is used by SiCl4 in forming adducts that gives SiCl4 this property?
A) sp2
B) sp3
C) sp2d
D) sp3d
E) sp3d2
A) sp2
B) sp3
C) sp2d
D) sp3d
E) sp3d2

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
22
Arsenic oxide (As2O3) is used as a standard in oxidation-reduction titrations because it is stable and reacts very predictably. Reaction with MnO4- in acidic solution gives aqueous arsenic (H3AsO4) and Mn2+. If the coefficient of MnO4-is 4 in the balanced chemical equation of reaction, the coefficient of H3AsO4 is
A) 2.
B) 4.
C) 5.
D) 8.
E) 10.
A) 2.
B) 4.
C) 5.
D) 8.
E) 10.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
23
Neutralization of 25 ml of 0.05 M H3PO4 requires what mass of solid sodium hydroxide, NaOH?
A) 0.05 g
B) 0.15 g
C) 0.02 g
D) 0.06 g
E) 2 g
A) 0.05 g
B) 0.15 g
C) 0.02 g
D) 0.06 g
E) 2 g

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
24
Which allotrope of carbon is the most thermodynamically stable?
A) diamond
B) fullerene
C) graphite
D) coke
E) carbon nanotube
A) diamond
B) fullerene
C) graphite
D) coke
E) carbon nanotube

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
25
CFCs, chlorofluorocarbons, are being phased out of use as refrigerants because
A) they highly reactive producing numerous pollutants in our atmosphere.
B) they are toxic.
C) they may remain in the atmosphere for hundreds of years, but react in the upper atmosphere.
D) they are gases and hard to contain.
E) hydrochlorfluorocarbons are cheaper and more effective refrigerants.
A) they highly reactive producing numerous pollutants in our atmosphere.
B) they are toxic.
C) they may remain in the atmosphere for hundreds of years, but react in the upper atmosphere.
D) they are gases and hard to contain.
E) hydrochlorfluorocarbons are cheaper and more effective refrigerants.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
26
When SF4 acts as a Lewis base, it donates an F- ion and generates the SF3+ ion. The structure of SF3+ is
A) T-shaped.
B) trigonal planar.
C) trigonal pyramidal.
D) tetrahedral.
E) distorted trigonal bipyramidal.
A) T-shaped.
B) trigonal planar.
C) trigonal pyramidal.
D) tetrahedral.
E) distorted trigonal bipyramidal.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
27
When SF4 acts as a Lewis acid, with trimethyl amine, it forms the adduct (CH3)3N-SF4. The structure of sulphur in (CH3)3N-SF4 is a(n)
A) distorted square planar.
B) tetrahedral.
C) octahedral.
D) square pyramidal.
E) trigonal bipyramidal.
A) distorted square planar.
B) tetrahedral.
C) octahedral.
D) square pyramidal.
E) trigonal bipyramidal.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
28
In the text, it is mentioned that sulphur compounds are poison for many catalysts. How, generally, would you expect that this is possible?
A) The sulphur coats the catalyst.
B) The sulphur reacts with the metal ions of the catalyst as a hard Lewis base.
C) The sulphur reacts with the other reactants.
D) The sulphur reacts with the metal ions of the catalyst as a soft Lewis base.
E) The sulphur changes the reaction products.
A) The sulphur coats the catalyst.
B) The sulphur reacts with the metal ions of the catalyst as a hard Lewis base.
C) The sulphur reacts with the other reactants.
D) The sulphur reacts with the metal ions of the catalyst as a soft Lewis base.
E) The sulphur changes the reaction products.

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck
29
Which polymer is fluoride found in?
A) CFC
B) Teflon
C) PVC
D) DDT
E) chlorofluorocarbons
A) CFC
B) Teflon
C) PVC
D) DDT
E) chlorofluorocarbons

Unlock Deck
Unlock for access to all 29 flashcards in this deck.
Unlock Deck
k this deck


