Deck 12: Spontaneity of Chemical Processes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/70
Play
Full screen (f)
Deck 12: Spontaneity of Chemical Processes
1
Recognize the driving force behind all chemical change: dispersal of energy and matter.
A process that is spontaneous in one direction is non-spontaneous in the opposite direction. Energy in the form of heat always flows from a warmer object to a colder one. The spontaneity of any process must be evaluated.
2
Predict the direction of change based on the entropy changes in the system and in the surroundings.
The second law of thermodynamics states that in any spontaneous process, the direction of change is such that total entropy increases: ∆Stotal > 0.
3
Understand and calculate entropies of pure substances.
The third law of thermodynamics states that a pure, perfect crystal at 0ºK has zero entropy. Entropy increases as the temperature increases, when a solid melts, and when a liquid evaporates.
4
Predict the direction of spontaneous change using the reaction free energy.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
5
Apply thermodynamics to chemical reactions and phase changes.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
6
Describe thermodynamically some representative energetic processes that operate in living organisms.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following arrangements of 4 identical socks in a 4-drawer dresser has the least degree of disorder?
A) 1 pair in 1 drawer and 1 pair in another drawer
B) 2 pair in 1 drawer
C) 1 pair in 1 drawer and 1 sock in 2 other drawers
D) 1 sock in each drawer
E) 3 socks in one drawer, 1 sock in another
A) 1 pair in 1 drawer and 1 pair in another drawer
B) 2 pair in 1 drawer
C) 1 pair in 1 drawer and 1 sock in 2 other drawers
D) 1 sock in each drawer
E) 3 socks in one drawer, 1 sock in another

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
8
Considerable energy is required to vapourize water, yet water does evaporate as demonstrated by clothes drying when hung out. The reason for this spontaneous process is
A) energy is always conserved.
B) an increase in disorder.
C) energy is released to the surroundings.
D) water has a higher vapour pressure outside rather than inside of a house.
E) an increase in order.
A) energy is always conserved.
B) an increase in disorder.
C) energy is released to the surroundings.
D) water has a higher vapour pressure outside rather than inside of a house.
E) an increase in order.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
9
The reaction shown below is exothermic:2 NO2 (g) N2O4(g)At low pressures it is NOT spontaneous, but at high pressures, significant amounts of product are formed. This is because
A) the heavier products cause greater disorder upon collisions.
B) the smaller number of product molecules leads to more disorder in the container.
C) to place the system under high pressure, the surroundings are more disordered.
D) the higher pressures make the reaction proceed more rapidly.
E) at low pressure more molecules are required to fill the same space.
A) the heavier products cause greater disorder upon collisions.
B) the smaller number of product molecules leads to more disorder in the container.
C) to place the system under high pressure, the surroundings are more disordered.
D) the higher pressures make the reaction proceed more rapidly.
E) at low pressure more molecules are required to fill the same space.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
10
The exothermic oxychlorination of ethylene to vinyl chloride is shown below:CH2CH2(g) + HCl(g) + O2(g) CH2CHCl(g) + H2O(g)What are the signs for the entropy change of the system and surroundings, respectively?
A) +ve, +ve
B) +ve, -ve
C) -ve,-ve
D) -ve, +ve
A) +ve, +ve
B) +ve, -ve
C) -ve,-ve
D) -ve, +ve

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
11
What are the signs for the entropy change of the system and surroundings, respectively, for ethane burning, when the system is defined as ethane and oxygen?
A) <0, <0
B) <0, >0
C) 0, 0
D) >0, <0
E) >0, >0
A) <0, <0
B) <0, >0
C) 0, 0
D) >0, <0
E) >0, >0

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
12
What are the signs for the entropy change of the system and surroundings, respectively, for water evaporating at 370ºK, when the system is defined as water?
A) <0, <0
B) <0, >0
C) 0, 0
D) >0, <0
E) >0, >0
A) <0, <0
B) <0, >0
C) 0, 0
D) >0, <0
E) >0, >0

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
13
Given three identical cups and three identical marbles, which of the following arrangements has the greatest value of W?
A) one marble in each cup
B) two marbles in one cup, one marble in one of the other cups
C) all three marbles in one cup
D) all have the same values of W
E) two of them have the same value of W
A) one marble in each cup
B) two marbles in one cup, one marble in one of the other cups
C) all three marbles in one cup
D) all have the same values of W
E) two of them have the same value of W

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following samples of substances has the smallest entropy change for fusion at its melting point, Tf? 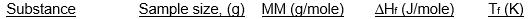
A)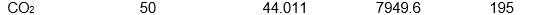
B)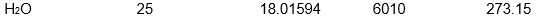
C)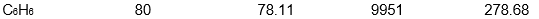
D)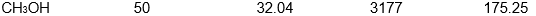
E)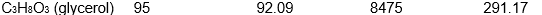
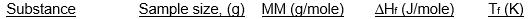
A)
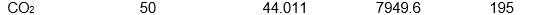
B)
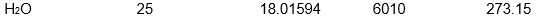
C)
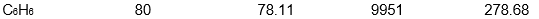
D)
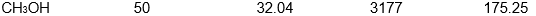
E)
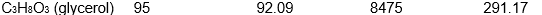

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following selections has the molecules arranged in order of increasing standard entropy? (One mole of each substance is being compared.)
A) C(graphite), C60(s), C(diamond)
B) Si(s), P4(s), S8(s)
C) C2H6(g), C2H4(g), CH4(g)
D) Polystyrene (made of 1000 monomer units), DNA (1000 base pairs), polyethylene (1000 monomer units)
E) CH4(g), C2H6(g), C2H4(g)
A) C(graphite), C60(s), C(diamond)
B) Si(s), P4(s), S8(s)
C) C2H6(g), C2H4(g), CH4(g)
D) Polystyrene (made of 1000 monomer units), DNA (1000 base pairs), polyethylene (1000 monomer units)
E) CH4(g), C2H6(g), C2H4(g)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
16
The standard molar entropy of NO is 211 J K-1 mol-1. What is the standard entropy of 3 moles of NO at 10 bar?
A) 202 J K-1 mol
B) 606 J K-1
C) 624 J K-1
D) 633 J K-1
E) 606 J K-1 mol-1
A) 202 J K-1 mol
B) 606 J K-1
C) 624 J K-1
D) 633 J K-1
E) 606 J K-1 mol-1

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
17
An important reagent in organic chemistry is thionyl chloride, SOCl2. One synthetic route would be to react SO2 with HCl in the gas phase to produce SOCl2 and water. What is the entropy change for this reaction? (J/K•mole SOCl2)? (S? = 309.7(SOCl2), 248.2(SO2), 186.9(HCl), 188.8(H2O) in J/K•mole)
A) -123
B) 123
C) -246
D) -146
E) 61.5
A) -123
B) 123
C) -246
D) -146
E) 61.5

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
18
One process water can undergo is dissociation, the equation for which is shown below:H2O (l) H+ (aq) + OH- (aq)As temperature increases to 90°C,
A) there will be no shift in the position of equilibrium.
B) more reactants will be present at equilibrium.
C) the extent of dissociation will be less.
D) the extent of dissociation will be greater.
E) the water will vapourize rather than dissociate.
A) there will be no shift in the position of equilibrium.
B) more reactants will be present at equilibrium.
C) the extent of dissociation will be less.
D) the extent of dissociation will be greater.
E) the water will vapourize rather than dissociate.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
19
A reaction will NEVER be spontaneous when
A) G° > 0.
B) H°< 0 and S° > 0.
C) H°< 0 and S° < 0.
D) G=0.
E) G > 0.
A) G° > 0.
B) H°< 0 and S° > 0.
C) H°< 0 and S° < 0.
D) G=0.
E) G > 0.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
20
Isomerization of hydrocarbons is important in the refining of petroleum. A simple example is that of butane, which has only two isomers: 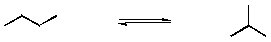
 Which of the following statements is most correct?
Which of the following statements is most correct?
A) At standard temperatures, n-butane is the preferred isomer.
B) At temperatures less than 450, n-butane is the preferred isomer.
C) At temperatures greater than 450ºK, n-butane is the preferred isomer.
D) At ambient temperatures, a sample of n-butane and isobutene which was at equilibrium would contain barely any butane.
E) A 1:1 mixture of butane and isobutane is at equilibrium at room temperature.
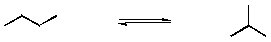
 Which of the following statements is most correct?
Which of the following statements is most correct?A) At standard temperatures, n-butane is the preferred isomer.
B) At temperatures less than 450, n-butane is the preferred isomer.
C) At temperatures greater than 450ºK, n-butane is the preferred isomer.
D) At ambient temperatures, a sample of n-butane and isobutene which was at equilibrium would contain barely any butane.
E) A 1:1 mixture of butane and isobutane is at equilibrium at room temperature.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
21
Consider the following chemical reaction:CaSO4(s) + 2H2O (g) CaSO4 - 2H2O (s)This reaction is exothermic.
A) The reaction is spontaneous at all temperatures.
B) The reaction is never spontaneous.
C) The reaction is spontaneous at low temperature.
D) The reaction is spontaneous at high temperature.
E) There is not sufficient information given to assess the spontaneity of the reaction.
A) The reaction is spontaneous at all temperatures.
B) The reaction is never spontaneous.
C) The reaction is spontaneous at low temperature.
D) The reaction is spontaneous at high temperature.
E) There is not sufficient information given to assess the spontaneity of the reaction.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
22
Consider the following unbalanced reaction:CO2(g) + H2O(g) (\rarr\) O2(g) + C3H8(g)This reaction is endothermic.
A) The reaction is spontaneous at all temperatures.
B) The reaction is never spontaneous.
C) The reaction is spontaneous at low temperature.
D) The reaction is spontaneous at high temperature.
E) There is not sufficient information given to assess the spontaneity of the reaction.
A) The reaction is spontaneous at all temperatures.
B) The reaction is never spontaneous.
C) The reaction is spontaneous at low temperature.
D) The reaction is spontaneous at high temperature.
E) There is not sufficient information given to assess the spontaneity of the reaction.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following energy producing technologies has no effect on the environment?
A) nuclear power
B) geothermal power
C) hydroelectric power
D) solar power
E) No energy producing technology is free from effects on the environment.
A) nuclear power
B) geothermal power
C) hydroelectric power
D) solar power
E) No energy producing technology is free from effects on the environment.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
24
A child plays with a deck of cards and leaves the room. You politely pick up and organize the cards. Which of the following is true during the action of organizing the cards?
A) You become more ordered.
B) The universe becomes more ordered.
C) The deck of cards becomes more disordered.
D) You become more disordered.
E) The ordering of a deck of cards is spontaneous.
A) You become more ordered.
B) The universe becomes more ordered.
C) The deck of cards becomes more disordered.
D) You become more disordered.
E) The ordering of a deck of cards is spontaneous.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following processes generate thermal pollution?
I. Hydroelectric generation of electricity
II. Nuclear generation of electricity
III. Coal powered generation of electricity
IV. Natural gas/oil powered generation of electricity
V. Wind farms
A) all of the above
B) I, II, III, and IV
C) III and IV
D) II, III, IV and V
E) II, III, IV
I. Hydroelectric generation of electricity
II. Nuclear generation of electricity
III. Coal powered generation of electricity
IV. Natural gas/oil powered generation of electricity
V. Wind farms
A) all of the above
B) I, II, III, and IV
C) III and IV
D) II, III, IV and V
E) II, III, IV

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
26
The process of storing energy for short term in the body is by making ATP. What type of bond is made or broken in this process?
A) making of a phosphate ester (P-O-P) bond
B) breaking of a phosphate ester bond
C) addition of water to ADP
D) the forming of a C=O bond
E) the forming of a C=C bond
A) making of a phosphate ester (P-O-P) bond
B) breaking of a phosphate ester bond
C) addition of water to ADP
D) the forming of a C=O bond
E) the forming of a C=C bond

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
27
How does your body perform non-spontaneous reactions?
A) coupling these reactions with spontaneous reactions
B) Non-spontaneous reactions do not occur in our body.
C) by directly using sugars or fats for energy for these reactions
D) by only using ADP
E) All reactions in the body are spontaneous.
A) coupling these reactions with spontaneous reactions
B) Non-spontaneous reactions do not occur in our body.
C) by directly using sugars or fats for energy for these reactions
D) by only using ADP
E) All reactions in the body are spontaneous.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
28
The following reaction has a Grxn = 2872 kJ/mol. How is it that this reaction happens in plants on a regular basis?
6 CO2(g) + 6 H2O(l) C6H12O6(aq) + 6 O2(g)
A) G says that the reaction is spontaneous.
B) This reaction is coupled with spontaneous reactions.
C) Energy is released to compensate for G.
D) This reaction happens in humans not plants.
E) It happens is small amounts so that not much energy is needed.
6 CO2(g) + 6 H2O(l) C6H12O6(aq) + 6 O2(g)
A) G says that the reaction is spontaneous.
B) This reaction is coupled with spontaneous reactions.
C) Energy is released to compensate for G.
D) This reaction happens in humans not plants.
E) It happens is small amounts so that not much energy is needed.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
29
Liquids composed of different molecules have different degrees of order. Compare the liquid phases of H2S and H2O and suggest which is more "ordered."

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
30
Ammonium nitrate, [NH4][NO3], dissolves spontaneously, even though the process is sufficiently endothermic to make it useful in "cold packs". Explain this phenomenon from the order-disorder perspective.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
31
State whether the disorder of each of the following systems increases or decreases in the stated process without consulting any tables:
i. 2 S (s) + 3 O2 (g) 2 SO3 (g)
ii. CO2 (g) CO2 (s)
iii. heating water from 5 °C to 75 °C
i. 2 S (s) + 3 O2 (g) 2 SO3 (g)
ii. CO2 (g) CO2 (s)
iii. heating water from 5 °C to 75 °C

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
32
State whether the disorder of each of the following systems increases or decreases in the stated process without consulting any tables:
i. 6 CO2(g) + 6 H2O(l) C6H12O6(aq) + 6 O2(g)
ii. CH4(g) + 2 O2(g) CO2(g) + 2 H2O(l)
iii. CH3CH2OH(l) CH3CH2OH(g)
i. 6 CO2(g) + 6 H2O(l) C6H12O6(aq) + 6 O2(g)
ii. CH4(g) + 2 O2(g) CO2(g) + 2 H2O(l)
iii. CH3CH2OH(l) CH3CH2OH(g)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
33
Compare the vapourization of H2S and H2O. For which of these will you expect the greater increase in disorder and why?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
34
What is the total entropy change when 40.5 g of ice ( Hfus = 6.01 kJ/mole) melts at 0oC?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
35
What is the total entropy change when 43 ml of water ( Hfus = 6.01 kJ/mole, density 0.9981 g ml-1) freezes at 0oC?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
36
Consider a 1 kg block of ice melting in a 35oC chamber ( Hfus = 6.01 kJ/mole). Determine the total entropy change for the ice and the chamber.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
37
Calculate the entropy change for the fusion of 1 mole of hydrogen sulphide, mp = -85.6°C and ∆Hfus = 2.39 kJ/mole.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
38
Calculate the entropy change of the universe for 1 mole of solid water melting at -5°C.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
39
What is the entropy change in the surroundings when the system is a 2.2 kg block of ice that melts in a swimming pool (surrounding) whose temperature is 31°C?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
40
What is the entropy change in the surroundings when a 25 g piece of ice, the system, melts in the mouth of a human (surrounding) which maintains a temperature of 37°C?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
41
Write the definition of the 3 Laws of Thermodynamics.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
42
What is the entropy change for the reaction of graphite (S˚=5.74 J/mol•K) and hydrogen (S˚=130.68) to produce methane (S˚=186.2)?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
43
In adjusting the acidity of swimming pool water, concentrated aqueous HCl is added. What is the entropy for the chloride ions after 0.5 L of 12.0 M HCl is added to a swimming pool of 9.6 x 104 L (about 25,000 gallons)? (S° Cl- (aq) = 56.5 J/mole • K)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
44
One mole of SF6 (g) (S°= 291.82 J/mole•K) is at STP. When released into a large room, the final concentration is 1 PPM by volume. What is the entropy change for the SF6 upon this expansion?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
45
Determine the change in entropy and if the following unbalanced reaction is spontaneous or non-spontaneous with respect to entropy.Ba(OH)2 8H2O(s) + NH4NO3(s) Ba(NO3)2(s) + H2O(l) + NH3(aq) 


Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
46
An electric heater releases 1200 J of energy every second. How much does the entropy of the surroundings increase when the heater runs for 10 minutes in a room that is at 25°C?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
47
Hydrogen sulphide is a toxic gas, which fortunately has the distinctive odour of rotten eggs. What is the entropy (J/K) of 0.01 mole of H2S (S?=205.8 J/K.mole) which occupies a volume of 106 L at a temperature of 298°K?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
48
Calculate the free energy for the oxychlorination of ethylene to vinyl chloride, CH2CHCl, under standard conditions.
G° = 53.6(CH2CHCl), -95.3(HCl), 68.49(CH2CH2), -228.7(H2O) kJ/molThe equation of reaction is:
CH2CH2 (g) + HCl (g) + EQ \F(1,2) O2 (g) CH2CHCl (g) + H2O (g)
G° = 53.6(CH2CHCl), -95.3(HCl), 68.49(CH2CH2), -228.7(H2O) kJ/molThe equation of reaction is:
CH2CH2 (g) + HCl (g) + EQ \F(1,2) O2 (g) CH2CHCl (g) + H2O (g)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
49
Calculate the free energy for the oxychlorination of ethylene to vinyl chloride, CH2CHCl, under standard conditions at 100oC given the thermodynamic data below collected at 25oC:
Go = 53.6(CH2CHCl), -95.3(HCl), 68.49(CH2CH2), -228.7(H2O) kJ/mol
Ho = 37.2(CH2CHCl), -92.3(HCl), 52.4(CH2CH2), -241.8(H2O) kJ/molThe equation of reaction is:
CH2CH2 (g) + HCl (g) + O2 (g) CH2CHCl (g) + H2O (g)
Go = 53.6(CH2CHCl), -95.3(HCl), 68.49(CH2CH2), -228.7(H2O) kJ/mol
Ho = 37.2(CH2CHCl), -92.3(HCl), 52.4(CH2CH2), -241.8(H2O) kJ/molThe equation of reaction is:
CH2CH2 (g) + HCl (g) + O2 (g) CH2CHCl (g) + H2O (g)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
50
The conversion of calcite,CaCO3(s), to CaO(s) and CO2(g) is NOT favourable at ambient temperatures. At what temperature will this decomposition of CaCO3 become spontaneous? 


Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
51
Determine if the following unbalanced reaction is spontaneous or non-spontaneous at standard conditions.
( G° = -857, SiO2; 0, Si; 0, Al; -1582 kJ/mol)
SiO2(s) + Al(s) Al2O3(s) + Si(s)
( G° = -857, SiO2; 0, Si; 0, Al; -1582 kJ/mol)
SiO2(s) + Al(s) Al2O3(s) + Si(s)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
52
What is the reaction quotient for the following unbalanced cell reaction?Ba(OH)2.08H2O(s) + NH4NO3(s) Ba(NO3)2(s) + H2O(l) + NH3(aq)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
53
Determine Gibb's Free Energy and if the following unbalanced reaction is spontaneous or non-spontaneous at standard conditions.
Ba(OH)2.8H2O(s) + NH4NO3(s) Ba(NO3)2(s) + H2O(l) + NH3(aq)
Ba(OH)2.8H2O(s) + NH4NO3(s) Ba(NO3)2(s) + H2O(l) + NH3(aq)


Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
54
Determine Gibb's Free Energy and if the following unbalanced reaction is spontaneous at 0oC.
Ba(OH)2.8H2O(s) + NH4NO3(s) Ba(NO3)2(s) + H2O(l) + NH3(aq)
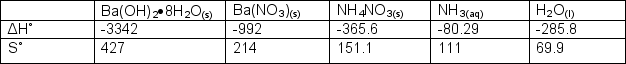
Ba(OH)2.8H2O(s) + NH4NO3(s) Ba(NO3)2(s) + H2O(l) + NH3(aq)


Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
55
At what identical concentration of H+ and CH3CO2- (?G°f = -372.5 kJ) will the dissociation of 1 M acetic acid, below, ( G°f (CH3CO2H (aq)) = -399.6 kJ) become spontaneous at room temperature?
CH3CO2H (aq) H+(aq) + CH3CO2-(aq)
CH3CO2H (aq) H+(aq) + CH3CO2-(aq)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
56
The possible isomerization for ethanol to methyl ether is shown below: 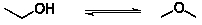
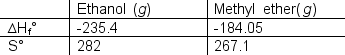
What is
a) the value of the reaction quotient when ?G=0 and
b) will the predominant species be ethanol or methyl ether?


What is
a) the value of the reaction quotient when ?G=0 and
b) will the predominant species be ethanol or methyl ether?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
57
Complete the following table for determining at what relative temperature (high or low, or at none) a reaction will be spontaneous.



Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
58
One of the more important industrial chemicals is hydrogen. One process for hydrogen production is called "steam reforming", in which hydrocarbons react with water to give hydrogen and CO. The equation of reaction for reforming methane is written below:
CH4 (g) + H2O (g) CO (g) + 3 H2 (g)
a) Calculate the free energy change for this reaction at standard conditions.
b) Estimate the temperature at which the process becomes spontaneous.
CH4 (g) + H2O (g) CO (g) + 3 H2 (g)

a) Calculate the free energy change for this reaction at standard conditions.
b) Estimate the temperature at which the process becomes spontaneous.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
59
Calcite, CaCO3(s), can be converted to CaO(s) and CO2(g). Determine the pressure of CO2 at 1150 K at which the reaction is no longer spontaneous. 


Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
60
One process for hydrogen production is called "steam reforming", in which hydrocarbons react with water to give hydrogen and CO. The equation of reaction for reforming methane is written below.
CH4 (g) + H2O (g) CO (g) + 3 H2 (g)
What is the free energy change, G, for reforming methane at 298oK when the partial pressure of the products is 5.0 x 10-5 atm and the partial pressure of the reactants is 1.0 atm?
CH4 (g) + H2O (g) CO (g) + 3 H2 (g)
What is the free energy change, G, for reforming methane at 298oK when the partial pressure of the products is 5.0 x 10-5 atm and the partial pressure of the reactants is 1.0 atm?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
61
What are the signs of ΔH, ΔS, and ΔG for the process of ice melting at 10˚C and 1 atm?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
62
Given the following chemical reaction at 298oK:3 O2(g) 2 O3(g) ?G? = 344 kJ/mole, what would be ?G if the partial pressure of O2 = 0.20 bar and O3 = 0.0001 bar?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
63
An explosive that is also used as fertilizer is ammonium nitrate. Calculate G for one of the decomposition reactions this fertilizer can undergo.
( G = -183.9 (NH4NO3), 103.7 (N2O), -228.7 (H2O) in kJ/mol)
NH4NO3(s) N2O(g) + 2 H2O(g)
( G = -183.9 (NH4NO3), 103.7 (N2O), -228.7 (H2O) in kJ/mol)
NH4NO3(s) N2O(g) + 2 H2O(g)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
64
At 45 C, what is the vapour pressure of Iodine?
(I2(s): H = 0, S = 116.1 J/mol.K) (I2(g): H = 62.4 kJ/mol, S = 260.7 J/mol.K)
(I2(s): H = 0, S = 116.1 J/mol.K) (I2(g): H = 62.4 kJ/mol, S = 260.7 J/mol.K)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
65
Estimate how many moles of ATP would be required to ride a bicycle at 35 km/hour for 1 hour, an activity that would require about 1500 kJ of energy, givenATP ADP + H2O, ?G° = -30.6 kJ

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
66
Glucose (MM = 180g/mole) is metabolized at a rate of about 38% efficiency. How many grams of glucose would supply the energy to ride a bicycle at 35 km/hour for 1 hour, an activity that would require about 1500 kJ of energy?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
67
Palmitic acid, C15H31CO2H undergoes complete combustion with Grxn o of -9790 kJ mol-1. Using bond energies, estimate the entropy change associated with this reaction.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
68
Chemical energy stored in glucose can be used to convert ADP to ATP; under normal physiological conditions the process, shown below, is 38.3 % efficient:
C6H12O6 + 6O2 + 36 ADP + 36 H3PO4 6CO2 + 36 ATP + 42 H2O G° = Given that the reaction: ADP + H3PO4 ATP + H2O requires 30.6 kJ mol-1 determine G° for the reaction of glucose and ADP above.
C6H12O6 + 6O2 + 36 ADP + 36 H3PO4 6CO2 + 36 ATP + 42 H2O G° = Given that the reaction: ADP + H3PO4 ATP + H2O requires 30.6 kJ mol-1 determine G° for the reaction of glucose and ADP above.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
69
The formation of ATP from ADP requires 30.6 kJ/mol. The oxidation of the fat, palmitic acid, to carbon dioxide yields -9790 kJ/mol of energy. Yet your body is only able to produce 130 ATP units per 1 palmitic acid unit. What is the percent yield for this biological process?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
70
A person exercises for 30 minutes and burns 800 kJ of free energy. How many moles of ATP (-30.6 kJ/mol) does this represent and based on 38% efficiency, how many grams of glucose were burned if 1 mole of glucose can make 36 ATPs?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck


