Deck 6: Fundamentals of Chemical Bonding
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/71
Play
Full screen (f)
Deck 6: Fundamentals of Chemical Bonding
1
Use the concept of electronegativity to determine the polarity of a chemical bond.
A covalent bond arises from the mutual attraction of the bonding electrons to the two nuclei. A greater difference in electronegativity leads to a more polar bond.
2
Draw optimized Lewis structures of covalent compounds, including resonance structures.
Only the valence electrons appear in a Lewis structure. Most atoms other than hydrogen are most stable when associated with an octet of electrons. The most likely Lewis structure has the lowest formal charges.
3
Recognize the importance of the tetrahedral shape in molecules.
An electron group can be two electrons in a single bond, four electrons in a double bond, six electrons in a triple bond, a pair of non-bonding electrons, or a single electron. The steric number of an inner atom is the sum of the number of ligands plus the number of lone pairs. Molecular shape describes how the ligands, not the electron groups, are arranged in space. Molecular shapes can be derived from the steric number and number of ligands bonded to a central atom.
4
Use the VSEPR model to predict the shapes of molecules with steric numbers 2, 3, 5, and 6.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
5
Understand the factors that influence bond angles, lengths, and energies.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is the most important component of formation of a H-Br bond?
A)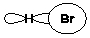
B)
C)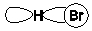
D)
E)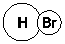
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following ranks the atoms in order of decreasing electronegativity?
A) Cl, O, Te, Mo, Rb
B) O, Cl, Mo, Rb, Te
C) Cl, O, Mo, Rb, Te
D) Rb, Mo, Te, Cl, O
E) O, Cl, Te, Mo, Rb
A) Cl, O, Te, Mo, Rb
B) O, Cl, Mo, Rb, Te
C) Cl, O, Mo, Rb, Te
D) Rb, Mo, Te, Cl, O
E) O, Cl, Te, Mo, Rb

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following ranks the atoms in order of increasing electronegativity?
A) F, Cl, O, C, Br
B) C, Br, Cl, O, F
C) F, O, Cl, Br, C
D) C, Br, O, Cl, F
E) C, O, Br, Cl, F
A) F, Cl, O, C, Br
B) C, Br, Cl, O, F
C) F, O, Cl, Br, C
D) C, Br, O, Cl, F
E) C, O, Br, Cl, F

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following ranks the following bonds from most polar to least polar?Cl-O, Mg-O, O-O, C-O
A) Mg-O>Cl-O>C-O>O-O
B) Cl-O>Mg-O> C-O>O-O
C) Cl-O> C-O> Mg-O> O-O
D) Mg-O> C-O> Cl-O>O-O
E) Mg-O>Cl-O>O-O> C-O
A) Mg-O>Cl-O>C-O>O-O
B) Cl-O>Mg-O> C-O>O-O
C) Cl-O> C-O> Mg-O> O-O
D) Mg-O> C-O> Cl-O>O-O
E) Mg-O>Cl-O>O-O> C-O

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following bonds is polar covalent?
A) LiF
B) Cl-Cl
C) Br-I
D) C-Cl
E) LiCl
A) LiF
B) Cl-Cl
C) Br-I
D) C-Cl
E) LiCl

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following bonds is ionic?
A) Li-F
B) Cu-Zn
C) Br-I
D) C-Cl
E) C-O
A) Li-F
B) Cu-Zn
C) Br-I
D) C-Cl
E) C-O

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following bonds is the least polar, but is still slightly polar?
A) Li-F
B) Fe-O
C) Br-I
D) C-C
E) C-I
A) Li-F
B) Fe-O
C) Br-I
D) C-C
E) C-I

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
13
Arrange the following in order of increasing electronegativity, C, O, F, Br, Ca.
A) C < O < F < Br < Ca
B) Ca < C < O < F < Br
C) Ca < C < Br < O < F
D) Ca < C < O < Br < F
E) Ca < O < C < Br < F
A) C < O < F < Br < Ca
B) Ca < C < O < F < Br
C) Ca < C < Br < O < F
D) Ca < C < O < Br < F
E) Ca < O < C < Br < F

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
14
How many valence electrons are in the molecule acetic acid, CH3CO2H?
A) 8
B) 12
C) 16
D) 20
E) 24
A) 8
B) 12
C) 16
D) 20
E) 24

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
15
How many valence electrons are in the phosphate ion, PO4-3?
A) 12
B) 24
C) 28
D) 32
E) 36
A) 12
B) 24
C) 28
D) 32
E) 36

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
16
What are the formal charges on the atoms in the following Lewis structure for HNO3? 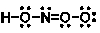
A) 1; 0; 0; 0; -1
B) 0; 0; 1; 0; -1
C) 1; 0; 1; 0; -2
D) 0; 0; 0; 0; 0
E) 0; 0; 0; 1; -1
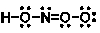
A) 1; 0; 0; 0; -1
B) 0; 0; 1; 0; -1
C) 1; 0; 1; 0; -2
D) 0; 0; 0; 0; 0
E) 0; 0; 0; 1; -1

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is NOT an important resonance structure for the nitrate ion?
A)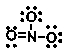
B)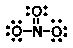
C)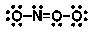
D)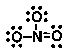
A)
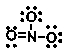
B)
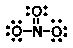
C)
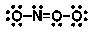
D)
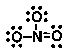

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
18
What can you change when drawing resonance structures?
A) total number of bonds
B) total number atoms
C) total number of electrons
D) total number of protons
E) nature of the bonds
A) total number of bonds
B) total number atoms
C) total number of electrons
D) total number of protons
E) nature of the bonds

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
19
How many valence electrons are there in the MnO4-?
A) 24
B) 32
C) 30
D) 28
E) 31
A) 24
B) 32
C) 30
D) 28
E) 31

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
20
Identify the central atom in the Lewis structure of thionyl chloride, SOCl2 and explain basis of decision.
A) O, because it is most electronegative
B) S, because it is least electronegative
C) S, because it is largest
D) Cl, because there are two
E) O, because it is smallest
A) O, because it is most electronegative
B) S, because it is least electronegative
C) S, because it is largest
D) Cl, because there are two
E) O, because it is smallest

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
21
What is the formal charge on Mn in the MnO4- ion? 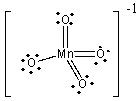
A) 0
B) 1
C) 3
D) 5
E) 7

A) 0
B) 1
C) 3
D) 5
E) 7

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following does not have a steric number of 4?
A) water
B) methane
C) ammonia
D) carbon dioxide
E) hydronium ion
A) water
B) methane
C) ammonia
D) carbon dioxide
E) hydronium ion

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
23
Hydridotributyltin, SnH(CH2CH2CH2CH3)3, is used in chemical syntheses. How many atoms in this molecule have tetrahedral geometry?
A) 5
B) 7
C) 9
D) 13
E) 15
A) 5
B) 7
C) 9
D) 13
E) 15

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
24
Dimethyl hydrazine, (CH3)2N-NH2, is a liquid at room temperature. How many atoms in this molecule have tetrahedral geometry?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
25
Dimethyl hydrazine, (CH3)2N-NH2, is a liquid at room temperature. How many atoms in this molecule have trigonal pyramidal geometry?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
26
How many ligands are around the nitrogen atom in the molecule methylamine, CH3NH2?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
27
What is the steric number for the central atom in the molecule iodine triflouride, IF3?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
28
What are the steric number and shape of thionyl chloride, SOCl2?
A) 3, trigonal planar
B) 3, trigonal pyramidal
C) 4, trigonal pyramidal
D) 4, square pyramidal
E) 4, tetrahedral
A) 3, trigonal planar
B) 3, trigonal pyramidal
C) 4, trigonal pyramidal
D) 4, square pyramidal
E) 4, tetrahedral

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
29
What is the molecular shape of the oxyanions of chlorine: ClO-, ClO2-, ClO3- and ClO4-?
A) bent, bent, trigonal planar, tetrahedral
B) linear, linear, trigonal planar, tetrahedral
C) linear, bent, trigonal planar, tetrahedral
D) linear, bent, trigonal pyramidal, tetrahedral
E) linear, linear, trigonal pyramidal, tetrahedral
A) bent, bent, trigonal planar, tetrahedral
B) linear, linear, trigonal planar, tetrahedral
C) linear, bent, trigonal planar, tetrahedral
D) linear, bent, trigonal pyramidal, tetrahedral
E) linear, linear, trigonal pyramidal, tetrahedral

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
30
What are the approximate bond angles in 1,2 dichloroethene?
A) 109.5
B) 90
C) 120
D) 115
E) 100
A) 109.5
B) 90
C) 120
D) 115
E) 100

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following compounds (CO2, O3, SO2, CS2) are polar?
A) CO2, SO2, CS2
B) CO2, O3, SO2, CS2
C) CO2, SO2
D) O3, CS2
E) O3, SO2
A) CO2, SO2, CS2
B) CO2, O3, SO2, CS2
C) CO2, SO2
D) O3, CS2
E) O3, SO2

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following compounds (CH4, CH3Cl, CH2Cl2, CHCl3 and CCl4) are polar?
A) CCl4, CHCl3, CH3Cl
B) CHCl3, CH3Cl
C) CHCl3, CH2Cl2, CH3Cl
D) CHCl3, CH2Cl2, CH3Cl, CCl4
E) CHCl3
A) CCl4, CHCl3, CH3Cl
B) CHCl3, CH3Cl
C) CHCl3, CH2Cl2, CH3Cl
D) CHCl3, CH2Cl2, CH3Cl, CCl4
E) CHCl3

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following molecules has a dipole moment?
A) SiCl4
B) AlI3
C) TeCl4
D) PCl5
E) CO2
A) SiCl4
B) AlI3
C) TeCl4
D) PCl5
E) CO2

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
34
In which of the following molecules will the bond angle be distorted from the ideal geometric values?1. SF5+2. ICl33. BrF54. Si(CH3)45. PF5
A) 1, 2, and 3
B) 3 and 5
C) 2, 3, and 4
D) 4 and 5
E) 2 and 3
A) 1, 2, and 3
B) 3 and 5
C) 2, 3, and 4
D) 4 and 5
E) 2 and 3

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following will have a dipole moment?
A) SF6
B) CH4
C) XeF4
D) PH3
E) C2H6
A) SF6
B) CH4
C) XeF4
D) PH3
E) C2H6

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
36
Which is the strongest bond?
A) P - P
B) P - F
C) P - Cl
D) P - Br
E) P - I
A) P - P
B) P - F
C) P - Cl
D) P - Br
E) P - I

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
37
Which is the strongest weakest?
A) P - P
B) P - F
C) P - Cl
D) P - Br
E) P - I
A) P - P
B) P - F
C) P - Cl
D) P - Br
E) P - I

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following will not have a dipole moment?
A) H2O
B) H2CO
C) CH3OH
D) SF6
E) SF4
A) H2O
B) H2CO
C) CH3OH
D) SF6
E) SF4

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is the strongest bond?
A)
B)
C)
D)
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
40
Draw a picture showing the interaction of the valence orbitals of lithium atoms in the formation of Li2.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
41
Draw a picture showing the interaction of the valence orbitals of Cl and Br in the formation of BrCl.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
42
Draw a diagram of energy vs. distance for the interaction of the H and Br atoms. Include sketches of the relative amounts of overlap of the orbitals. The bond energy of HBr is 363 kJ/mole and the H-Br bond length is 140.8 pm.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
43
Sketch the overlap of the valance orbitals in HF.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
44
Identify the bonding, non-bonding, and core orbitals in HBr.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
45
Calculate the electronegativity difference between the atoms in the bonds found below and predict which atom would be positively charged:
O = 3.5, N = 3.0, Cl = 3.0, C = 2.5,
Al = 1.5, Cs = 0.7
(i) N-O
(ii) Al-C
(iii) Cl-Cs
O = 3.5, N = 3.0, Cl = 3.0, C = 2.5,
Al = 1.5, Cs = 0.7
(i) N-O
(ii) Al-C
(iii) Cl-Cs

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
46
Draw the Lewis structure of ionic compound sodium sulphite, Na2SO3.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
47
Draw the Lewis structure of dichlorodifluoromethane, CF2Cl2.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
48
Draw the Lewis structure and determine the formal charge on the central atom for BF5.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
49
Draw the three most important resonance structures for the carbonate ion, CO32-.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
50
Draw 2 important resonance structures of the thiocyanate ion, NCS-1.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
51
Draw 3 resonance structures for N2O, otherwise known as laughing gas.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
52
Draw Lewis structure of KF2.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
53
Draw the Lewis structure for thionyl chloride, SOCl2.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
54
Draw the Lewis structure of methylamine, H3C-NH2.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
55
Draw the two structural isomers of C2H3Cl3.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
56
Draw a ball and stick model that shows the structure of methylamine, H3CNH2.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
57
Hydroxylamine has the formula NH2(OH). Write the Lewis structure and state the molecular structure of the inner atoms.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
58
Hydrogen sulphide has the formula H2S. Write the Lewis structure and state the structure of the inner atoms.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
59
Draw the Lewis structure and state the structure of the inner atoms for ClF3.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
60
Draw the Lewis structure and state the molecular structure of the inner atoms for BrF5.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
61
Write the Lewis structure and state the molecular structure of the inner atoms for SeCl4.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
62
Write the Lewis structure and state the molecular structure of the inner atoms for N2O4.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
63
Write the Lewis structure and state the molecular structure of the inner atoms for AlCl3.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
64
Write the Lewis structure and state the molecular structure of the inner atoms for XeO2F4.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
65
Write the Lewis structure and state the molecular structure of the inner atoms for O3.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
66
Draw the two structural isomers of C2H3Cl3 and determine which isomer has the greater dipole moment.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
67
Is the oxygen - oxygen bond longer in molecular oxygen, O2 or ozone, O3? Explain.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
68
Draw the Lewis structure and estimate the bond angles for bromine pentafluoride, BrF5.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
69
Draw the Lewis structure and estimate the bond angles for phosphorous trichloride, PCl3.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
70
Draw the Lewis structure and estimate the bond angles in methanol, CH3OH.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the molecules, NH3 or PH3, should have the stronger bond and explain why.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck


