Deck 5: Atomic Energies and Periodicity
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/71
Play
Full screen (f)
Deck 5: Atomic Energies and Periodicity
1
Explain the effects of nuclear charge and screening on the energies of electrons.
The higher the value of the quantum number l, the more that orbital is screened by electrons in smaller, more stable orbitals.
2
Understand the relationships between the structure of the periodic table and electron configurations.
The Pauli exclusion principle states that each electron in an atom has a unique set of quantum numbers. The Aufbau principle states that electrons are placed into atomic orbitals beginning with the lowest-energy electrons, followed by successively higher-energy electrons. Valence electrons are all those of highest principal quantum number plus those in partially filled d and f orbitals.
3
Use the Pauli exclusion principle, Hund's rule, and the orbital filling order to predict electron configurations of atoms and ions.
The most stable configuration involving orbitals of equal energies is the one with the maximum number of electrons with the same spin orientation (Hund's rule).
4
Relate trends in atomic radius, ionization energy, and electron affinity to nuclear charge and electron configuration.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
5
Understand why ionic compounds exist and the energetics of their formation.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
6
Understand the trends in atomic radius, ionization energy, and electron affinity and their relationships to nuclear charge and electron configuration.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
7
Consider the atomic orbitals of indium. Which of the following statements are true?
1) The 5p orbital completely screens the 5s orbital.
2) The 5s orbital is more stable than the 5p orbital.
3) The orbitals with n = 4 or less shield the 5s orbital.
4) The 5s orbital is less stable than the 5p orbital.
5) The 5s orbital completely screens the 5p orbital.
A) 1 and 3
B) 3, 4 and 5
C) 2, 3 and 5
D) 2 and 3
E) 1 and 4
1) The 5p orbital completely screens the 5s orbital.
2) The 5s orbital is more stable than the 5p orbital.
3) The orbitals with n = 4 or less shield the 5s orbital.
4) The 5s orbital is less stable than the 5p orbital.
5) The 5s orbital completely screens the 5p orbital.
A) 1 and 3
B) 3, 4 and 5
C) 2, 3 and 5
D) 2 and 3
E) 1 and 4

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
8
The energies for removal of a 1s electron from a H atom, a He atom and a He+ ion are 2.18x10-18 J, 3.94x10-18 J, and 8.72x10-18 J, respectively. These values indicate
A) ionization energies directly reflect the nuclear charge.
B) partially shielding results from electrons having the same principle and azimuthal quantum numbers.
C) s electrons effectively shield p electrons of the same principal quantum number.
D) shielding results only from electrons having lower principal quantum number.
E) ionization energy is independent of nuclear charge.
A) ionization energies directly reflect the nuclear charge.
B) partially shielding results from electrons having the same principle and azimuthal quantum numbers.
C) s electrons effectively shield p electrons of the same principal quantum number.
D) shielding results only from electrons having lower principal quantum number.
E) ionization energy is independent of nuclear charge.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
9
The ionization energy for a H atom 1s electron is 2.18x10-18 J and that for a 2p electron is 0.545x10-18J.
A) The 2p electron is shielded by the 1s electron, and as a result IE is lower.
B) A 2p electron is further from the nucleus than a 1s electron would be, and as a result IE is lower.
C) The 2p electron is shielded by the 1s electron and will be more easily removed.
D) The 1s electron is closer to the nucleus than a 2p electron would be, therefore it is easier to remove the 1s electron.
E) Electrons never occupy 2p orbitals in hydrogen.
A) The 2p electron is shielded by the 1s electron, and as a result IE is lower.
B) A 2p electron is further from the nucleus than a 1s electron would be, and as a result IE is lower.
C) The 2p electron is shielded by the 1s electron and will be more easily removed.
D) The 1s electron is closer to the nucleus than a 2p electron would be, therefore it is easier to remove the 1s electron.
E) Electrons never occupy 2p orbitals in hydrogen.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
10
The 2s orbital calcium is more stable than the 2p orbital even though the 2p orbital has its maximum electron density closer to the nucleus. The reason for this higher stability is
A) calcium's valence orbitals have n = 4.
B) the 1s orbital screens the 2p orbital more than the 2s orbital.
C) the 2s orbital has more electron density closer to the nucleus than the 2p orbital.
D) calcium is an exception to usual trends.
E) all s orbitals are more stable than p orbitals.
A) calcium's valence orbitals have n = 4.
B) the 1s orbital screens the 2p orbital more than the 2s orbital.
C) the 2s orbital has more electron density closer to the nucleus than the 2p orbital.
D) calcium is an exception to usual trends.
E) all s orbitals are more stable than p orbitals.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
11
The energy required to remove an electron from a helium ion, He+, (8.72 x 10-18 J) is more than twice the energy required to remove an electron from a helium atom (3.94 x 10-18 J). The reason(s) are
A) 1s electrons screen each other.
B) the helium ion has a larger Z.
C) the helium atom has less electron-electron repulsions.
D) the measurement is compromised by the Heisenberg uncertainty principle.
E) helium ions have smaller nuclei.
A) 1s electrons screen each other.
B) the helium ion has a larger Z.
C) the helium atom has less electron-electron repulsions.
D) the measurement is compromised by the Heisenberg uncertainty principle.
E) helium ions have smaller nuclei.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
12
Titanium atoms have electrons in 1s, 2s, 2p, 3s, 3p, 4s, and 3d orbitals. Which of the following has the orbitals listed in the order of decreasing screening ability?
A) 1s, 2p, 2s, 3p
B) 2s, 2p, 3d, 3s
C) 2s, 2p, 3s, 3d
D) 1s, 2s, 3p, 3s
E) 3s, 4s, 3p, 3d
A) 1s, 2p, 2s, 3p
B) 2s, 2p, 3d, 3s
C) 2s, 2p, 3s, 3d
D) 1s, 2s, 3p, 3s
E) 3s, 4s, 3p, 3d

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
13
Screening accounts for
A) electron-electron repulsions.
B) different numbers of neutrons and protons in the nucleus.
C) differences in deBroglie wavelength.
D) the nuclear charge of different atoms.
E) the nuclear charge actually experienced by an electron.
A) electron-electron repulsions.
B) different numbers of neutrons and protons in the nucleus.
C) differences in deBroglie wavelength.
D) the nuclear charge of different atoms.
E) the nuclear charge actually experienced by an electron.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
14
Consider the following statements on screening. Which of the following statements are true?
A) The amount of screening depends only on the size of the orbital.
B) The amount of screening depends only on the shape of the orbital.
C) Electrons occupying orbitals of the same principal quantum number do not screen one another effectively.
D) Electrons with the same azimuthal quantum number but different values of the magnetic quantum number do not screen one another effectively.
E) Electrons in d and f orbitals do not screen electrons in s orbitals.
A) The amount of screening depends only on the size of the orbital.
B) The amount of screening depends only on the shape of the orbital.
C) Electrons occupying orbitals of the same principal quantum number do not screen one another effectively.
D) Electrons with the same azimuthal quantum number but different values of the magnetic quantum number do not screen one another effectively.
E) Electrons in d and f orbitals do not screen electrons in s orbitals.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
15
The theory that states that no 2 electrons on an atom can have the same set of quantum numbers was written by
A) Hund.
B) Pauli.
C) Avogadro.
D) Aufbau.e( Heisenberg.
A) Hund.
B) Pauli.
C) Avogadro.
D) Aufbau.e( Heisenberg.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
16
Which orbital(s) is/are partially filled in chromium atoms?
A) 4s
B) 3p
C) 3s
D) 3d
E) a and d
A) 4s
B) 3p
C) 3s
D) 3d
E) a and d

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
17
What is the maximum number of electrons that can occupy the orbitals with principal quantum number = 4?
A) 2
B) 8
C) 18
D) 32
E) 14
A) 2
B) 8
C) 18
D) 32
E) 14

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
18
What is the number of electrons that can occupy an orbital with principal quantum number = 4?
A) 2
B) 8
C) 18
D) 32
E) 14
A) 2
B) 8
C) 18
D) 32
E) 14

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
19
What is the maximum number of electrons that can occupy the orbitals with azimuthal quantum number = 3?
A) 7
B) 14
C) 5
D) 10
E) 6
A) 7
B) 14
C) 5
D) 10
E) 6

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following atoms have six valence electrons?
O, W, Se, Eu, Nd
A) 1 and 3
B) 1, 2, and 3
C) 1, 3 and 4
D) 1, 2, 3 and 5
E) 1, 2, 3, and 4
O, W, Se, Eu, Nd
A) 1 and 3
B) 1, 2, and 3
C) 1, 3 and 4
D) 1, 2, 3 and 5
E) 1, 2, 3, and 4

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following atoms have three valence electrons?
1) B
2) Li
3) Y
4) Element 104
5) Hf
A) 1 and 4
B) 1, 2, and 3
C) 1, 3 and 5
D) 3 and 5
E) 1 and 5
1) B
2) Li
3) Y
4) Element 104
5) Hf
A) 1 and 4
B) 1, 2, and 3
C) 1, 3 and 5
D) 3 and 5
E) 1 and 5

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following have 4 valance electrons?
A) Al
B) Si
C) P
D) As
E) Be
A) Al
B) Si
C) P
D) As
E) Be

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
23
Which orbitals are partially filled in Zr atoms?
A) 5s
B) 4p
C) 4s
D) 4d
E) a and d
A) 5s
B) 4p
C) 4s
D) 4d
E) a and d

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
24
What is the maximum number of electrons that can occupy the orbitals with principal quantum number = 3?
A) 2
B) 8
C) 18
D) 6
E) 10
A) 2
B) 8
C) 18
D) 6
E) 10

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
25
The electron configuration [Ar]4s13d5 is
A) an excited state configuration of Cr.
B) the ground state configuration of Cr.
C) the ground state electron configuration of Mn.
D) an excited state electron configuration of Mn.
E) the ground state electron configuration of Mo.
A) an excited state configuration of Cr.
B) the ground state configuration of Cr.
C) the ground state electron configuration of Mn.
D) an excited state electron configuration of Mn.
E) the ground state electron configuration of Mo.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is the electron configuration of ground state As atoms?
A) [Ar]4s3d104p4
B) [Ar]3s23d103p3
C) [Ar]4s23d104p3
D) [Ar]4s23d104p4
E) [Ar]4s24p63d7
A) [Ar]4s3d104p4
B) [Ar]3s23d103p3
C) [Ar]4s23d104p3
D) [Ar]4s23d104p4
E) [Ar]4s24p63d7

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is the electron configuration of ground state Nb atoms?
A) [Kr]5s24d4
B) [Kr]5s14d2
C) [Kr]5s14d3
D) [Kr]5s24d1
E) [Kr]5s24d3
A) [Kr]5s24d4
B) [Kr]5s14d2
C) [Kr]5s14d3
D) [Kr]5s24d1
E) [Kr]5s24d3

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
28
What is the electron configuration of a silicon atom?
A) 1s22s23s22p63p2
B) 1s22s23s22p63p4
C) 1s22s22p63s23p2
D) 1s22s22p63p4
E) 1s22s22p63s4
A) 1s22s23s22p63p2
B) 1s22s23s22p63p4
C) 1s22s22p63s23p2
D) 1s22s22p63p4
E) 1s22s22p63s4

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
29
What is the electron configuration of vanadium using the noble gas configuration?
A) [Ne]4s24d3
B) [Ar]4s23d3
C) [Ar]3d5
D) [Ar]4s24d3
E) [Ar]4s24p3
A) [Ne]4s24d3
B) [Ar]4s23d3
C) [Ar]3d5
D) [Ar]4s24d3
E) [Ar]4s24p3

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
30
How many unpaired electrons are in a molybdenum atom?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
31
How many unpaired electrons are on a sulphur atom?
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
32
How many pairs of valence electrons exist on a Cl-1 ion?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
33
Salts of Gd3+ are used in magnetic resonance imaging to enhance the quality of the image. How many unpaired electrons are in a Gd3+ ion?
A) 0
B) 4
C) 6
D) 7
E) 9
A) 0
B) 4
C) 6
D) 7
E) 9

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
34
How many unpaired electrons are there on a Mn+2 ion?
A) 3
B) 4
C) 5
D) 6
E) 7
A) 3
B) 4
C) 5
D) 6
E) 7

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
35
How many unpaired electrons are there on a Co+2 ion?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
36
The two outermost electrons of ground state carbon can be described by quantum numbers (n, l, ml,ms)
A) (2,1,1,+1/2) and (2,1,1,-1/2)
B) (2,1,1,+1/2) and (2,1,0,+1/2)
C) (2,0,0,+1/2) and (2,0,0,-1/2)
D) (2,0,0,+1/2) and (2,1,0,+1/2)
E) (2,1,1,+1/2) and (2,1,0,-1/2)
A) (2,1,1,+1/2) and (2,1,1,-1/2)
B) (2,1,1,+1/2) and (2,1,0,+1/2)
C) (2,0,0,+1/2) and (2,0,0,-1/2)
D) (2,0,0,+1/2) and (2,1,0,+1/2)
E) (2,1,1,+1/2) and (2,1,0,-1/2)

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is NOT an excited state of Si?
A)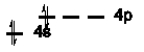
B)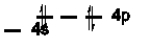
C)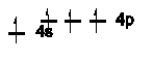
D)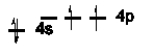
E)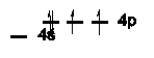
A)
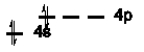
B)
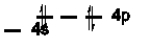
C)
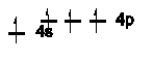
D)
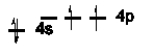
E)
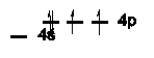

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
38
Sulphur is smaller than phosphorus because
A) it has fewer unpaired electrons.
B) sulphur's electrons screen more effectively.
C) the effective nuclear charge of sulphur is larger.
D) the two elements are in the same row.
E) it is in the same period and further to the right in the periodic table.
A) it has fewer unpaired electrons.
B) sulphur's electrons screen more effectively.
C) the effective nuclear charge of sulphur is larger.
D) the two elements are in the same row.
E) it is in the same period and further to the right in the periodic table.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following choices has the ions in order of decreasing size?
A) Rb+, Br-, Se2-
B) Br-, Se2-, Rb+
C) Br-, Rb+, Se2-
D) Se2-, Br-, Rb+
E) Rb+, Se2-, Br-
A) Rb+, Br-, Se2-
B) Br-, Se2-, Rb+
C) Br-, Rb+, Se2-
D) Se2-, Br-, Rb+
E) Rb+, Se2-, Br-

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is in the order of increasing ionization energy?
A) I, P, Cl
B) I, Cl, P
C) P, Cl, I
D) Cl, P, I
E) P, I, Cl
A) I, P, Cl
B) I, Cl, P
C) P, Cl, I
D) Cl, P, I
E) P, I, Cl

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
41
Ionization energy decreases going down a family (column) because
A) valence orbitals become more stable.
B) screening becomes more effective.
C) orbitals are larger.
D) electron affinities are smaller.
E) number of protons in the nucleus increase.
A) valence orbitals become more stable.
B) screening becomes more effective.
C) orbitals are larger.
D) electron affinities are smaller.
E) number of protons in the nucleus increase.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
42
The main reason for the anomalous positive electron affinity of nitrogen atoms is
A) electron-electron repulsion in the nitrogen atom.
B) electron-electron repulsion in the nitrogen ion, N-.
C) a lower than expected screening in the N atom.
D) increased negative ionic charge.
E) electrons are strongly attracted to the nucleus.
A) electron-electron repulsion in the nitrogen atom.
B) electron-electron repulsion in the nitrogen ion, N-.
C) a lower than expected screening in the N atom.
D) increased negative ionic charge.
E) electrons are strongly attracted to the nucleus.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following is expected to have the highest ionization energy for the next electron?
A) F
B) Sc2+
C) Ca2+
D) Al3+
E) Ga3+
A) F
B) Sc2+
C) Ca2+
D) Al3+
E) Ga3+

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
44
Which answer has the elements arranged in order of least to most negative electron affinity?
A) Br, Cl, F
B) N, O, F
C) Na, Mg, F
D) C, N, F
E) F, N, O
A) Br, Cl, F
B) N, O, F
C) Na, Mg, F
D) C, N, F
E) F, N, O

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
45
Which answer, has the elements arranged in order of most negative to least negative electron affinity?
A) Ge, As, Se
B) As, Ge, Se
C) As, Se, Ge
D) Se, As, Ge
E) Se, Ge, As
A) Ge, As, Se
B) As, Ge, Se
C) As, Se, Ge
D) Se, As, Ge
E) Se, Ge, As

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following has the lowest ionization energy?
A) Mg2+
B) Na+
C) O2-
D) Se2-
E) F-
A) Mg2+
B) Na+
C) O2-
D) Se2-
E) F-

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following has the greatest magnitude?
A) the first ionization energy of strontium
B) the first electron affinity of fluorine
C) the second ionization energy of magnesium
D) the first ionization energy of oxygen
E) the third ionization energy of magnesium
A) the first ionization energy of strontium
B) the first electron affinity of fluorine
C) the second ionization energy of magnesium
D) the first ionization energy of oxygen
E) the third ionization energy of magnesium

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
48
The primary reason that an ionic compound of formula KCl2 does NOT form is
A) the lattice energy is smaller than that of KCl.
B) the electron affinity of Cl is endothermic.
C) the bond energy of Cl2 is prohibitively large.
D) the total ionization energy to reach K+2 is too high.
E) the second electron affinity of Cl is positive.
A) the lattice energy is smaller than that of KCl.
B) the electron affinity of Cl is endothermic.
C) the bond energy of Cl2 is prohibitively large.
D) the total ionization energy to reach K+2 is too high.
E) the second electron affinity of Cl is positive.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
49
The primary reason that an ionic compound of formula K2Cl does NOT form is
A) the lattice energy is smaller than that of KCl.
B) the first electron affinity of Cl is endothermic.
C) the bond energy of Cl2 is prohibitively large.
D) the total ionization energy to reach K+2 is too high.
E) the second electron affinity of Cl is positive.
A) the lattice energy is smaller than that of KCl.
B) the first electron affinity of Cl is endothermic.
C) the bond energy of Cl2 is prohibitively large.
D) the total ionization energy to reach K+2 is too high.
E) the second electron affinity of Cl is positive.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
50
The lattice energy of CaCl
A) is much larger than as that of KCl.
B) is smaller than that of CaCl2.
C) is larger than that of NaCl.
D) is independent of the size of the cation.
E) is much smaller than that of KCl.
A) is much larger than as that of KCl.
B) is smaller than that of CaCl2.
C) is larger than that of NaCl.
D) is independent of the size of the cation.
E) is much smaller than that of KCl.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
51
Consider the following elements:Si, Co, Cl, P, Sr, F, Rb. Select the most accurate statement.
A) Rb, S,r and Co will form ions of +2 charge.
B) Al, Si, P, and Cl will form stable anions.
C) Co, Sr, and Rb will form ionic compounds with Cl and F.
D) Sr and Si will form ionic compounds with Co.
E) Rb, Sr, and Co will form ions of +1 charge.
A) Rb, S,r and Co will form ions of +2 charge.
B) Al, Si, P, and Cl will form stable anions.
C) Co, Sr, and Rb will form ionic compounds with Cl and F.
D) Sr and Si will form ionic compounds with Co.
E) Rb, Sr, and Co will form ions of +1 charge.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
52
Which ionic compound will have the largest lattice energy?
A) NaCl
B) LiCl
C) KCl
D) CsCl
E) RbCl
A) NaCl
B) LiCl
C) KCl
D) CsCl
E) RbCl

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
53
The alkali metals are found in nature as ionic compounds because
A) it takes very little energy to remove an s electron.
B) the electron affinity for these alkali metals is negative.
C) lattice energy is sufficient to overcome the energy required to form the cation.
D) the cations have a noble gas electron configuration.
E) second ionization energies are very large and positive.
A) it takes very little energy to remove an s electron.
B) the electron affinity for these alkali metals is negative.
C) lattice energy is sufficient to overcome the energy required to form the cation.
D) the cations have a noble gas electron configuration.
E) second ionization energies are very large and positive.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
54
Which ionic compound will have the smallest lattice energy?
A) KCl
B) CaCl2
C) FeCl3
D) MgCl2
E) NaCl
A) KCl
B) CaCl2
C) FeCl3
D) MgCl2
E) NaCl

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
55
Which ionic compound will have the largest lattice energy?
A) Li2O
B) MgO
C) FeCl3
D) MgCl2
E) Fe2O3
A) Li2O
B) MgO
C) FeCl3
D) MgCl2
E) Fe2O3

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following compounds is lime?
A) MgO
B) CaOH
C) MgOH
D) CaO
E) CaCO3
A) MgO
B) CaOH
C) MgOH
D) CaO
E) CaCO3

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following compounds is potash?
A) MgO
B) CaO
C) K2O
D) KOH
E) Ca5(PO4)F
A) MgO
B) CaO
C) K2O
D) KOH
E) Ca5(PO4)F

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
58
Carbonates contain which of the following anions?
A) CO2-
B) CO3-
C) CO32-
D) HCO3-
E) HCO2
A) CO2-
B) CO3-
C) CO32-
D) HCO3-
E) HCO2

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
59
Typically the second row of nonmetals, (C and N) make oxoanions with how many oxygen atoms?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
60
Boron has the following photelectron spectrum. Based on this draw what the photoelectron spectrum for carbon should be. 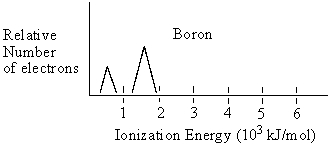


Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
61
What is the definition of a "valence electron"?

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
62
Draw the ground state energy diagram for the valence electrons of selenium.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
63
Draw the ground state energy level diagram for Cu2+.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
64
Why do the atoms follow the general trend of becoming smaller as they move across a row?

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
65
Explain why MgN would NOT be expected to be stable.

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
66
Using a Born-Haber analysis like that shown in the text, estimate the energy released on forming KCl2 from K(s)and Cl2 where K has a +2 charge (the lattice energy for KCl2 is about 2200 kJ/mole; heat of vaporization (K)= 77.1 kJ/mole; IE1(K) =419 kJ/mole; IE2(K)=3051 kJ/mole; Cl2 bond energy = 240 kJ/mole; EA(Cl)= -348.8 kJ).

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
67
What is the major use of sodium carbonate?

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
68
Why do the s and d block elements readily form cations?

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the group 15 elements is metallic?

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the group 14 elements are metalloids?

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck
71
Which groups of the periodic table contain no metals?

Unlock Deck
Unlock for access to all 71 flashcards in this deck.
Unlock Deck
k this deck


