Deck 3: Energy and Its Conservation
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/65
Play
Full screen (f)
Deck 3: Energy and Its Conservation
1
Recognize the types of energy of interest to chemists.
NOT ANSWER
2
Understand the first law of thermodynamics and the concepts of heat and work.
Energy is neither created nor destroyed in any process, although it may be transferred from one body to another or transformed from one form into another. The value of a state function does not depend on the path taken or on the rate of the change.
3
Understand the origins of energy changes in chemical reactions.
Breaking bonds is endothermic. Making bonds is exothermic.
4
Apply the principles of calorimetry to determine energy changes in a chemical reaction.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
5
Understand and calculate enthalpy and internal energy.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
6
Be familiar with our sources of energy.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
7
Use the following data for Questions :
Table 3-1; Table 3-2; q = nC?T; w = Force*distance; ?E = q + w
-Al2O3 is the raw material for manufacturing aluminum and must be heated considerably before it can be converted to aluminum. Calculate the amount of heat (in kJ) required to heat 200 kg of Al2O3 (C = 79.04 J/molK) from 25°C to 1000°C and compare that to the amount of heat required to heat 200 kg of Al metal (C = 24.35 J/molK) from 25°C to 550°C. What is the difference?
A) 9.25 x 105 kJ
B) 9.47 x 104 kJ
C) 7407 kJ
D) 79.04 kJ
E) 1961 kJ
Table 3-1; Table 3-2; q = nC?T; w = Force*distance; ?E = q + w
-Al2O3 is the raw material for manufacturing aluminum and must be heated considerably before it can be converted to aluminum. Calculate the amount of heat (in kJ) required to heat 200 kg of Al2O3 (C = 79.04 J/molK) from 25°C to 1000°C and compare that to the amount of heat required to heat 200 kg of Al metal (C = 24.35 J/molK) from 25°C to 550°C. What is the difference?
A) 9.25 x 105 kJ
B) 9.47 x 104 kJ
C) 7407 kJ
D) 79.04 kJ
E) 1961 kJ

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
8
Use the following data for Questions :
Table 3-1; Table 3-2; q = nC?T; w = Force*distance; ?E = q + w
-A CRC Handbook of Chemistry and Physics has a mass of about 2.83 kg. How much work must be done against gravity to lift the book from the floor to the shelf 2.0 m off the floor? (Note: the force due to gravity is Fg = m(9.8 m/s2) where m is the mass of the object.)
A) 20 J
B) 40 J
C) 55 J
D) 70 J
E) 85 J
Table 3-1; Table 3-2; q = nC?T; w = Force*distance; ?E = q + w
-A CRC Handbook of Chemistry and Physics has a mass of about 2.83 kg. How much work must be done against gravity to lift the book from the floor to the shelf 2.0 m off the floor? (Note: the force due to gravity is Fg = m(9.8 m/s2) where m is the mass of the object.)
A) 20 J
B) 40 J
C) 55 J
D) 70 J
E) 85 J

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
9
Use the following data for Questions :
Table 3-1; Table 3-2; q = nC?T; w = Force*distance; ?E = q + w
-A refrigerator uses a compressor to do work to "remove the heat" from warm things within it. A foam cup containing 150 g of water at 23.0°C is placed in a refrigerator. What is the minimum work the compressor must do to cool the water to 1.5°C?
A) 1.2 kJ
B) 5.6 kJ
C) 13 kJ
D) 18 kJ
E) 56 kJ
Table 3-1; Table 3-2; q = nC?T; w = Force*distance; ?E = q + w
-A refrigerator uses a compressor to do work to "remove the heat" from warm things within it. A foam cup containing 150 g of water at 23.0°C is placed in a refrigerator. What is the minimum work the compressor must do to cool the water to 1.5°C?
A) 1.2 kJ
B) 5.6 kJ
C) 13 kJ
D) 18 kJ
E) 56 kJ

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
10
Use the following data for Questions :
Table 3-1; Table 3-2; q = nC?T; w = Force*distance; ?E = q + w
-A 5.6 g bullet travelling at 650 m/s is stopped upon impact with a pool of water. How much heat is released?
A) 400 J
B) 600 J
C) 1000 J
D) 1200 J
E) 1500 J
Table 3-1; Table 3-2; q = nC?T; w = Force*distance; ?E = q + w
-A 5.6 g bullet travelling at 650 m/s is stopped upon impact with a pool of water. How much heat is released?
A) 400 J
B) 600 J
C) 1000 J
D) 1200 J
E) 1500 J

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
11
Use the following data for Questions :
Table 3-1; Table 3-2; q = nC?T; w = Force*distance; ?E = q + w
-How many kJ of energy will be converted to heat when a 1.4 x 103 kg automobile stops from a speed of 67 km/hr?
A) 240 kJ
B) 265 kJ
C) 290 kJ
D) 315 kJ
E) 400 kJ
Table 3-1; Table 3-2; q = nC?T; w = Force*distance; ?E = q + w
-How many kJ of energy will be converted to heat when a 1.4 x 103 kg automobile stops from a speed of 67 km/hr?
A) 240 kJ
B) 265 kJ
C) 290 kJ
D) 315 kJ
E) 400 kJ

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
12
Nylon is an important component in many products. One step in the production of nylon is the reaction of cyclohexane with oxygen to give cyclohexanone and water, shown below in the balanced reaction in line formula notation. Determine the energy change for this process using bond energies. (See Table 3-2.) 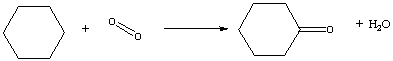
A) ?E = -246 kJ/mol
B) ?E = 246 kJ/mol
C) ?E = -346 kJ/mol
D) ?E = 346 kJ/mol
E) ?E = -305 kJ/mol

A) ?E = -246 kJ/mol
B) ?E = 246 kJ/mol
C) ?E = -346 kJ/mol
D) ?E = 346 kJ/mol
E) ?E = -305 kJ/mol

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
13
Estimate ?E for the following reaction using bond energies: (See Table 3-2 in the text)
C2H4(g) + Br2 (g) C2H4Br2 (g)
A) -275 kJ/mol
B) -90 kJ/mol
C) 90 kJ/mol
D) 275 kJ/mol
E) -360 kJ/mol
C2H4(g) + Br2 (g) C2H4Br2 (g)
A) -275 kJ/mol
B) -90 kJ/mol
C) 90 kJ/mol
D) 275 kJ/mol
E) -360 kJ/mol

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
14
Based on bond energies, determine the reaction energy per mole of carbon for C2H4. (See Table 3-2 in the text )
A) -1280 kJ mol-1
B) -640 kJ mol-1
C) 640 kJ mol-1
D) -1550 kJ mol-1
E) 775 kJ mol-1
A) -1280 kJ mol-1
B) -640 kJ mol-1
C) 640 kJ mol-1
D) -1550 kJ mol-1
E) 775 kJ mol-1

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
15
The reaction of ethene, C2H4, to form polyethylene is shown below: (See Table 3-2 in the text) 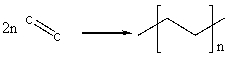 Estimate how much energy is released when one mole of ethene forms polyethylene using bond energies.
Estimate how much energy is released when one mole of ethene forms polyethylene using bond energies.
A) 69 kJ/mol
B) 75 kJ/mol
C) 345 kJ/mol
D) 690 kJ/mol
E) 38 kJ/mol
 Estimate how much energy is released when one mole of ethene forms polyethylene using bond energies.
Estimate how much energy is released when one mole of ethene forms polyethylene using bond energies.A) 69 kJ/mol
B) 75 kJ/mol
C) 345 kJ/mol
D) 690 kJ/mol
E) 38 kJ/mol

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
16
The combustion of an organic compound releases 35.6 kJ of energy and causes the temperature of a constant volume calorimeter to increase by 4.76°C. What is the heat capacity of the calorimeter? (C = 75.291 J/mol.K for H2O; q = Ccal T)
A) 3.05 kJ/K
B) 5.67 kJ/K
C) 6.90 kJ/K
D) 7.48 kJ/K
E) 12.3 kJ/K
A) 3.05 kJ/K
B) 5.67 kJ/K
C) 6.90 kJ/K
D) 7.48 kJ/K
E) 12.3 kJ/K

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
17
A constant pressure "coffee-cup" calorimeter is used to measure the heat of reaction between 0.10 L of 1.0 M NaOH and 0.10 L of 1.0 M HCl. Both solutions initially are at 24.0°C; after the solutions are mixed, the final temperature is 30.4°C. If the heat capacity of the resulting solution is assumed to be totally due to water (Cp = 75.291 J/mol•K), how much heat was evolved from the reaction per mole of limiting reactant? Assume the density of all solutions is 1.0 g/mL. (C = 75.291 J/mol.K for H2O; q = Ccal T)
A) 5.4 kJ/mol
B) 22 kJ/mol
C) 54 kJ/mol
D) 123 kJ/mol
E) 270 kJ/mol
A) 5.4 kJ/mol
B) 22 kJ/mol
C) 54 kJ/mol
D) 123 kJ/mol
E) 270 kJ/mol

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
18
At 11:30 pm at a large university on a night during finals week, 7500 students each use an electrical heater to heat 450 g of water from 20°C to 100°C to make a cup of coffee. How many kJ of electrical energy is consumed to make their beverages? Assume 100% efficiency.(C = 75.291 J/mol . K for H2O; q = Ccal T)
A) 110 kJ
B) 150 kJ
C) 1.5 x 105 kJ
D) 1.1 x 106 kJ
E) 1.1 x 109 kJ
A) 110 kJ
B) 150 kJ
C) 1.5 x 105 kJ
D) 1.1 x 106 kJ
E) 1.1 x 109 kJ

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
19
A constant volume calorimeter's temperature increases by 2.34 K when a standard compound is combusted, releasing 2.14 kJ of energy. A 0.5623 g sample of sucrose, C12H22O11, is then combusted in the same calorimeter and a temperature increase of 10.13 K is noted. What is the energy released per mole of sucrose combusted?(C = 75.291 J/mol . K for H2O; q = Ccal T)
A) 5623 kJ/mol
B) 5640 kJ/mol
C) 8,434 kJ/mol
D) 10,355 kJ/mol
E) 3168 kJ/mol
A) 5623 kJ/mol
B) 5640 kJ/mol
C) 8,434 kJ/mol
D) 10,355 kJ/mol
E) 3168 kJ/mol

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
20
How much energy is required to heat 450 g of water from 20°C to 100°C?(C = 75.291 J/mol . K for H2O; q = Ccal T)
A) 1.5 x 103 J
B) 3.6 x 104 J
C) 4.5 x 103 J
D) 1.5 x 102 kJ
E) 3.6 x 102 kJ
A) 1.5 x 103 J
B) 3.6 x 104 J
C) 4.5 x 103 J
D) 1.5 x 102 kJ
E) 3.6 x 102 kJ

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
21
Lactic acid (line structure below) is an intermediate in the metabolism of glucose.(C = 75.291 J/mol.K for H2O; q = Ccal T) 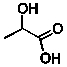 When 0.149 g of lactic acid is combusted in a constant volume calorimeter in an excess of oxygen, only CO2 and water are products and 2.24 kJ of energy is released. If the heat capacity of the calorimeter is 0.827 kJ/K and the initial temperature was 23.44°C, what is the final temperature in °C? (C = 75.291 J/mol.K for H2O; q = Ccal T)
When 0.149 g of lactic acid is combusted in a constant volume calorimeter in an excess of oxygen, only CO2 and water are products and 2.24 kJ of energy is released. If the heat capacity of the calorimeter is 0.827 kJ/K and the initial temperature was 23.44°C, what is the final temperature in °C? (C = 75.291 J/mol.K for H2O; q = Ccal T)
A) 2.71°C
B) 26.15°C
C) 25.11°C
D) 27.23°C
E) 28.34°C
 When 0.149 g of lactic acid is combusted in a constant volume calorimeter in an excess of oxygen, only CO2 and water are products and 2.24 kJ of energy is released. If the heat capacity of the calorimeter is 0.827 kJ/K and the initial temperature was 23.44°C, what is the final temperature in °C? (C = 75.291 J/mol.K for H2O; q = Ccal T)
When 0.149 g of lactic acid is combusted in a constant volume calorimeter in an excess of oxygen, only CO2 and water are products and 2.24 kJ of energy is released. If the heat capacity of the calorimeter is 0.827 kJ/K and the initial temperature was 23.44°C, what is the final temperature in °C? (C = 75.291 J/mol.K for H2O; q = Ccal T)A) 2.71°C
B) 26.15°C
C) 25.11°C
D) 27.23°C
E) 28.34°C

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
22
How much energy in kJ must be absorbed to heat up a volume of ocean water 2 m high by 20 km wide by 30 km deep by 3°C? (The density of sea water is about 1.02 g/mL; it requires about 4.2 J to heat up 1 g of sea water 1 K.) (C = 75.291 J/mol.K for H2O; q = Ccal T)
A) 1.2 x 1010 kJ
B) 5.1 x 1012 kJ
C) 9.8 x 1012 kJ
D) 1.5 x 1013 kJ
E) 9.8 x 1015 kJ
A) 1.2 x 1010 kJ
B) 5.1 x 1012 kJ
C) 9.8 x 1012 kJ
D) 1.5 x 1013 kJ
E) 9.8 x 1015 kJ

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
23
How much energy must be removed to cool a volume of air 1 km high by 20 km wide x 30 km deep by 5°C? (The density of air is about 1.29 g/L; it requires 1.02 J to heat up 1 g of air 1K.) (C = 75.291 J/mol.K for H2O; q = Ccal T)
A) 1.4 x 1010 kJ
B) 2.6 x 1012 kJ
C) 4.0 x 1012 kJ
D) 9.3 x 1012 kJ
E) 4.0x109 kJ
A) 1.4 x 1010 kJ
B) 2.6 x 1012 kJ
C) 4.0 x 1012 kJ
D) 9.3 x 1012 kJ
E) 4.0x109 kJ

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
24
A 1.72 g sample of the saccharide, C6H12O6, shown below is combusted in a constant volume calorimeter of heat capacity 8.21 kJ/K. The initial temperature is 22.1°C. 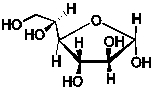 The final temperature is 25.4°C. What is the energy released per mole of this sugar upon combustion? (C = 75.291 J/mol.K for H2O; q = Ccal T)
The final temperature is 25.4°C. What is the energy released per mole of this sugar upon combustion? (C = 75.291 J/mol.K for H2O; q = Ccal T)
A) 2830 kJ/mol
B) 3530 kJ/mol
C) 4565 kJ/mol
D) 5865 kJ/mol
E) 4868 kJ/mol
 The final temperature is 25.4°C. What is the energy released per mole of this sugar upon combustion? (C = 75.291 J/mol.K for H2O; q = Ccal T)
The final temperature is 25.4°C. What is the energy released per mole of this sugar upon combustion? (C = 75.291 J/mol.K for H2O; q = Ccal T)A) 2830 kJ/mol
B) 3530 kJ/mol
C) 4565 kJ/mol
D) 5865 kJ/mol
E) 4868 kJ/mol

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
25
A piece of cauliflower was combusted in a calorimeter with a heat capacity of 5.24 kJ/°C. The temperature increased from 24.35°C to 25.07°C. What was the enthalpy change for the combustion of the cauliflower? (C = 75.291 J/mol.K for H2O; q = Ccal T)
A) 3.8 kJ
B) -3.8 kJ
C) 6.5 kJ
D) -6.5 kJ
E) -7.3 kJ
A) 3.8 kJ
B) -3.8 kJ
C) 6.5 kJ
D) -6.5 kJ
E) -7.3 kJ

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
26
What is the work (per mole of water) associated with the chemical process in which hydrogen and oxygen gases combine to form liquid water at 25 oC?
( Hrxn = Hproducts - Hreactants; Hrxn = Bond broken - Bonds made)
A) +3.7 kJ/mol
B) -3.7 kJ/mol
C) +1.2 kJ/mol
D) -1.2 kJ/mol
E) 7.4 kJ/mol
( Hrxn = Hproducts - Hreactants; Hrxn = Bond broken - Bonds made)
A) +3.7 kJ/mol
B) -3.7 kJ/mol
C) +1.2 kJ/mol
D) -1.2 kJ/mol
E) 7.4 kJ/mol

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
27
The oxidation of sulphur dioxide to sulphur trioxide is important in the manufacture of sulphuric acid:
2 SO2 (g) + O2 (g) 2 SO3 (g)
Find the enthalpy change of this reaction given the following:
S (s) + O2 (g) SO2 (g); H° = -296.83 kJ
2 S (s) + 3 O2 (g) 2 SO3 (g); H° = -791.44 kJ
( Hrxn = Hproducts - Hreactants; Hrxn = Bond broken - Bonds made)
A) -197.78 kJ
B) 197.78 kJ
C) -485.23 kJ
D) 791.22 kJ
E) -1088.27 kJ
2 SO2 (g) + O2 (g) 2 SO3 (g)
Find the enthalpy change of this reaction given the following:
S (s) + O2 (g) SO2 (g); H° = -296.83 kJ
2 S (s) + 3 O2 (g) 2 SO3 (g); H° = -791.44 kJ
( Hrxn = Hproducts - Hreactants; Hrxn = Bond broken - Bonds made)
A) -197.78 kJ
B) 197.78 kJ
C) -485.23 kJ
D) 791.22 kJ
E) -1088.27 kJ

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
28
Determine H for the following reaction:C2H2(g) + 2 H2(g) C2H6(g); H =? given the following heats of combustion:
C2H2 + 5/2 O2 2 CO2 + H2O H = -1300 kJ
H2 + 1/2 O2 H2O H = -286 kJ
C2H6 + 7/2 O2 2 CO2 + 3 H2O H = -1560 kJ
( Hrxn = Hproducts - Hreactants; Hrxn = Bond broken - Bonds made)
A) -154 kJ
B) -256 kJ
C) -312 kJ
D) -648 kJ
E) -3146 kJ
C2H2 + 5/2 O2 2 CO2 + H2O H = -1300 kJ
H2 + 1/2 O2 H2O H = -286 kJ
C2H6 + 7/2 O2 2 CO2 + 3 H2O H = -1560 kJ
( Hrxn = Hproducts - Hreactants; Hrxn = Bond broken - Bonds made)
A) -154 kJ
B) -256 kJ
C) -312 kJ
D) -648 kJ
E) -3146 kJ

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
29
The enthalpy and energy changes for a chemical reaction are
A) never the same number.
B) often the same number.
C) are the same when there is no volume change.
D) are the same when all reactants and products are gases.
E) are different when all reactants and products are solids.
A) never the same number.
B) often the same number.
C) are the same when there is no volume change.
D) are the same when all reactants and products are gases.
E) are different when all reactants and products are solids.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
30
What is the difference between H and E for the reaction at 25oC of O(g) + N(g) NO(g)?
( Hrxn = Hproducts - Hreactants; Hrxn = Bond broken - Bonds made)
A) 2.5 kJ
B) -2.5 kJ
C) 0.2 kJ
D) -0.2 kJ
E) 0 kJ
( Hrxn = Hproducts - Hreactants; Hrxn = Bond broken - Bonds made)
A) 2.5 kJ
B) -2.5 kJ
C) 0.2 kJ
D) -0.2 kJ
E) 0 kJ

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
31
What is the difference between H and E per mole of product, for the reaction at 25 oC of O2(g) + N2(g) 2NO(g)?
( Hrxn = Hproducts - Hreactants; Hrxn = Bond broken - Bonds made)
A) 5.0 kJ
B) -5.0 kJ
C) 2.5 kJ
D) -2.5 kJ
E) 0 kJ
( Hrxn = Hproducts - Hreactants; Hrxn = Bond broken - Bonds made)
A) 5.0 kJ
B) -5.0 kJ
C) 2.5 kJ
D) -2.5 kJ
E) 0 kJ

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
32
Assume that coal is essentially all carbon and has a density of 1.5 g/mL.1. How much heat is produced if a piece of coal 7cm x 5 cm x 6 cm undergoes complete combustion by the following reaction:
C (s) + O2 (g) CO2 (g); H = -394 kJ2.
What mass of water could be heated from 25°C to 100°C with this amount of heat?
( Hrxn = Hproducts - Hreactants; Hrxn = Bond broken - Bonds made)
A) -1.0 x104 kJ, 3.3 x104g of H2O
B) +1.0 x104kJ, 33g of H2O
C) 5.0 x103kJ, 33 g H2O
D) -5.0 x103kJ, 3,300g of H2O
E) -1.0 x104 kJ, 1.8 x103g of H2O
C (s) + O2 (g) CO2 (g); H = -394 kJ2.
What mass of water could be heated from 25°C to 100°C with this amount of heat?
( Hrxn = Hproducts - Hreactants; Hrxn = Bond broken - Bonds made)
A) -1.0 x104 kJ, 3.3 x104g of H2O
B) +1.0 x104kJ, 33g of H2O
C) 5.0 x103kJ, 33 g H2O
D) -5.0 x103kJ, 3,300g of H2O
E) -1.0 x104 kJ, 1.8 x103g of H2O

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is the equation corresponding to the formation reaction for nitric acid in the gas phase? ( Hrxn = Hproducts - Hreactants; Hrxn = Bond broken - Bonds made)
A) H2 + N2 + O2 HNO3
B) H (g) + N(g) + 3 O (g) HNO3 (g)
C) H (g) + N(g) + O3 (g) HNO3 (g)
D) 1/2 H2 (g) + 1/2 N2(g) + 3/2 O2 (g) HNO3 (g)
E) H2 (g) + N2(g) + 3 O2 (g) 2 HNO3 (g)
A) H2 + N2 + O2 HNO3
B) H (g) + N(g) + 3 O (g) HNO3 (g)
C) H (g) + N(g) + O3 (g) HNO3 (g)
D) 1/2 H2 (g) + 1/2 N2(g) + 3/2 O2 (g) HNO3 (g)
E) H2 (g) + N2(g) + 3 O2 (g) 2 HNO3 (g)

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
34
Calculate the standard enthalpy change for the combustion of lactic acid, C3H6O3.Given: Hf° (lactic acid) = -671 kJ/mole; Hf H2O = -242.83; CO2 = -393.5; O2 = 0 kJ/mol.( Hrxn = Hproducts - Hreactants; Hrxn = Bond broken - Bonds made)
A) 345 kJ/mol
B) -656 kJ/mol
C) 1238 kJ/mol
D) -1238 kJ/mol
E) -5346 kJ/mol
A) 345 kJ/mol
B) -656 kJ/mol
C) 1238 kJ/mol
D) -1238 kJ/mol
E) -5346 kJ/mol

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
35
Calculate the standard enthalpy change for the manufacture of HCl by the following reaction:2 NaCl (s) + H2SO4 (l) 2 HCl (g) + Na2SO4 (s)Given: Hf (kJ/mol)= -411.2 (NaCl), -814 (H2SO4), -92.3 (HCl), -1387.1 (Na2SO4).( Hrxn = Hproducts - Hreactants; Hrxn = Bond broken - Bonds made)
A) 64.7 kJ
B) -64.7 kJ
C) -456 kJ
D) 456 kJ
E) -1190 kJ
A) 64.7 kJ
B) -64.7 kJ
C) -456 kJ
D) 456 kJ
E) -1190 kJ

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
36
Where does the earth get most of its energy from?
A) aliens
B) sunlight
C) solar winds
D) gravitational friction
E) nuclear power
A) aliens
B) sunlight
C) solar winds
D) gravitational friction
E) nuclear power

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
37
Where do we hope to get most of our energy from in the future?
A) fossil fuels
B) coal
C) biomass
D) hydroelectric dams
E) gasoline
A) fossil fuels
B) coal
C) biomass
D) hydroelectric dams
E) gasoline

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
38
In the following processes, identify the type of energy consumed and the type of energy produced. (chemical, electrical, mechanical, gravitational, kinetic, potential, radiant, and/or thermal):
1. A piece of metal is heated and glows bright red.
2. A "lightstick" is bent and begins to glow.
3. A car crashes into a pole and the body metal is warmed.
4. Methane is burned and a hot air balloon rises into the sky.
1. A piece of metal is heated and glows bright red.
2. A "lightstick" is bent and begins to glow.
3. A car crashes into a pole and the body metal is warmed.
4. Methane is burned and a hot air balloon rises into the sky.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
39
How are the following phenomena consistent with the conservation of energy?
1. A bouncing ball bounces lower each consecutive bounce.
2. A solar panel absorbs light and produces electricity.
3. A car gets worse gas mileage going uphill than on the flats.
1. A bouncing ball bounces lower each consecutive bounce.
2. A solar panel absorbs light and produces electricity.
3. A car gets worse gas mileage going uphill than on the flats.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
40
What is the kinetic energy of a 1000 kg car moving at 30 meters per second? What is the kinetic energy of the same car moving at 60 meters per second? What does this suggest about the distance it takes for the faster car to be stopped by its brakes?

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
41
What is the kinetic energy of a 114,000 kg jet moving at 750 mph?

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
42
What is the kinetic energy of a proton moving at 90% of the speed of light? (massproton = 1.67262 x10-27 kg)

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
43
What is the mass of a jet plane with a kinetic energy of 6.40 x 109 J moving at 750 miles per hour?

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
44
What is the velocity of a jet plane that has a mass of 114,000 kg and kinetic energy of 9.56 x 108 J?

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
45
Determine the electrical potential energy between two electrons separated by 100pm.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
46
Determine the electrical potential energy between a proton and an electron separated by 100pm.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
47
What is the kinetic energy of an electron with a velocity of 2.87 x106 m/s? (masselectron = 9.109 x10-31 kg)

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
48
Is water in a sealed glass tube a closed or isolated system? Give a brief explanation.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
49
Is an ice cube with water in a perfect, stoppered thermos an open, closed or isolated system? Give a brief explanation. (Perfect implies that the thermos allows no energy transfer to or from the interior of the thermos.)

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
50
100 g samples of each silver, aluminum, mercury, copper and iron (molar heat capacities of 25.351, 24.35, 27.983, 24.435 and 25.10 J mol-1 C-1, respectively) are subjected to 200 kJ of heat. Which samples sees the largest increase in temperature?

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
51
Indicate the sign of the thermodynamic quantities in the appropriate box for the given system by placing a mark in the appropriate box: 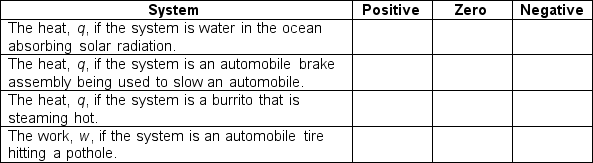


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
52
Determine the change in energy of a system when an engine does 25 kJ of work and gives off 30 kJ of heat.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
53
Determine the change in energy of a system when an engine does 30 kJ of work while absorbing 40 kJ of heat.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
54
Determine the change in energy of a system that absorbs 80 kJ of energy and does 30 kJ of work and releases 25 kJ of heat.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
55
The solvent dichloromethane, CH2Cl2, is made from the reaction of methane, CH4, with chlorine gas, Cl2. The other product of the reaction is hydrogen chloride, HCl. Draw a molecular picture representing the balanced chemical reaction for this process (showing all chemical bonds) and then determine the energy change for the reaction of one mole of methane to give one mole of dichloromethane using bond enthalpies.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
56
Using bond energies determine which of CH4, C2H4 and C2H6 results in the greatest reaction energy per mole of carbon on combustion to CO2 and water.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
57
Determine the difference in energy between the complete combustion of ethane, C2H6 to CO2 and the incomplete combustion of ethane to CO (base on bond energies, assume bond energy of CO is 1080 kJ mol-1).

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
58
Benzene, C6H6, is a compound whose Lewis structure has three double bonds. Estimate the energy change upon hydrogenation using bond energies, and calculate the % error, given the true energy change, ?E = -169 kJ/mole. 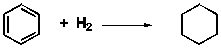
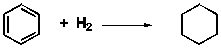

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
59
An experiment in a coffee cup calorimeter results in qrxn = -6 kJ. Is the reaction exothermic or endothermic and will the calorimeter temperature increase or decrease?

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
60
When 0.891 g of terephthalic acid (line structure below) is combusted in excess oxygen, 17.3 kJ of energy is released. What is the balanced chemical equation for the combustion of terephthalic acid and the energy released per mole upon combustion? 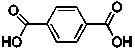


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
61
A 50 g sample of an unknown metal (listed in the below table) is heated to 100°C and placed in 100 g of water at 22.1°C. The final temperature is 29.0°C. Which of the metals listed is the unknown? 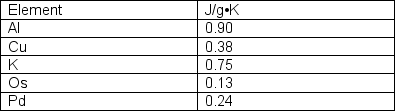


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
62
A 1200 gram sample of iron is heated to 85 ?C. It is then placed in a bowl that contains 600 grams of water at 18 ?C. What will be the final temperature once equilibrium is established assuming no heat is lost to the surrounding? (CFe = 25.10 J/mol.K, Cwater = 75.291 J/mol.K)

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
63
Write the chemical equation of the reaction whose enthalpy change is the standard enthalpy of formation of AlCl3 (g).

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
64
A liquid is vaporized at its boiling point. Is this an exothermic process or endothermic?

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
65
A sample of water is frozen in your refrigerator. Determine if each part of the system is exothermic or endothermic; the water, the refrigerator, the entire system (water and refrigerator).

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck


