Deck 1: Fundamental Concepts of Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/118
Play
Full screen (f)
Deck 1: Fundamental Concepts of Chemistry
1
Recognize elemental symbols and names of the elements, and name compounds from molecular pictures.
Properties of molecules (the microscopic level) translate into properties of materials (the macroscopic level).
2
Recognize the SI units commonly used in chemistry, and perform some common unit conversions.
The correct conversion ratio leads to cancellation of unwanted units. Proper use of significant figures is important to tell the reader what the accuracy and precision of the measurement are.
3
Analyze and solve problems in a consistent, organized fashion.Solving Quantitative ProblemsStep 1. Determine what is asked for.Step 2. Visualize the problem.Step 3. Organize the data.Step 4. Identify a process to solve the problem.Step 5. Manipulate the equations.Step 6. Substitute and calculate.Step 7. Does the result make sense?
NOT ANSWER
4
Solve mass-number-molar mass-type problems.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
5
Perform mole-mass-number conversions.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
6
Calculate concentrations of solutions and of diluted solutions.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
7
Balance chemical reactions.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
8
Calculate the amount of a product from the amounts of the reactants and a balanced chemical equation.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
9
Calculate yields of chemical reactions.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
10
Solve limiting-reagent-type problems.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is a microscopic property?
A) the colour of a substance
B) the density of a substance
C) the arrangement of atoms in the molecules making up the substance
D) the mass of a substance
E) the shape of the crystals in a solid substance
A) the colour of a substance
B) the density of a substance
C) the arrangement of atoms in the molecules making up the substance
D) the mass of a substance
E) the shape of the crystals in a solid substance

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
12
What is the correct formula for molecular oxygen?
A) O
B) 2O
C) O2
D) O2
E) O3
A) O
B) 2O
C) O2
D) O2
E) O3

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
13
Use the following information for Questions
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) ?r3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1
yard = 3 ft; 1 gal = 3.785 L; ?C = 5/9 (?F - 32)
proton charge = +1.6022 x 10-19 C mass = 1.6726 x 10-27 kg
electron charge = -1.6022 x 10-19 C mass = 9.1094 x 10-31 kg
neutron charge = 0 mass = 1.6749 x 10-27 kg
-Which of the following are valid conversions from cubic yards to mm3?
A)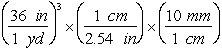
B)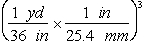
C)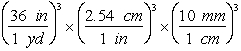
D)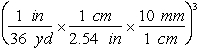
E)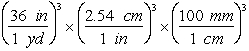
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) ?r3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1
yard = 3 ft; 1 gal = 3.785 L; ?C = 5/9 (?F - 32)
proton charge = +1.6022 x 10-19 C mass = 1.6726 x 10-27 kg
electron charge = -1.6022 x 10-19 C mass = 9.1094 x 10-31 kg
neutron charge = 0 mass = 1.6749 x 10-27 kg
-Which of the following are valid conversions from cubic yards to mm3?
A)
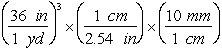
B)
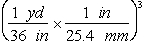
C)
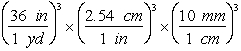
D)
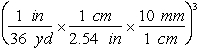
E)
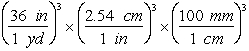

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
14
Use the following information for Questions
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) ?r3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1
yard = 3 ft; 1 gal = 3.785 L; ?C = 5/9 (?F - 32)
proton charge = +1.6022 x 10-19 C mass = 1.6726 x 10-27 kg
electron charge = -1.6022 x 10-19 C mass = 9.1094 x 10-31 kg
neutron charge = 0 mass = 1.6749 x 10-27 kg
-One less common temperature unit is the Rankine. This scale has the same magnitude degree increment as the Fahrenheit scale, but zero Rankine is the same as absolute zero, or 0 K. Absolute zero is -459.7° Fahrenheit. Which of the following are true?
A) Water freezes at 32 Rankine and boils at 212 Rankine.
B) Water freezes at 273 Rankine and boils at 373 Rankine.
C) Room temperature, 25°C, is 536.7 Rankine.
D) Room temperature, 25°C, is 563.7 Rankine.
E) Body temperature, 37°C, is 563.7 Rankine.
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) ?r3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1
yard = 3 ft; 1 gal = 3.785 L; ?C = 5/9 (?F - 32)
proton charge = +1.6022 x 10-19 C mass = 1.6726 x 10-27 kg
electron charge = -1.6022 x 10-19 C mass = 9.1094 x 10-31 kg
neutron charge = 0 mass = 1.6749 x 10-27 kg
-One less common temperature unit is the Rankine. This scale has the same magnitude degree increment as the Fahrenheit scale, but zero Rankine is the same as absolute zero, or 0 K. Absolute zero is -459.7° Fahrenheit. Which of the following are true?
A) Water freezes at 32 Rankine and boils at 212 Rankine.
B) Water freezes at 273 Rankine and boils at 373 Rankine.
C) Room temperature, 25°C, is 536.7 Rankine.
D) Room temperature, 25°C, is 563.7 Rankine.
E) Body temperature, 37°C, is 563.7 Rankine.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
15
Use the following information for Questions
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) ?r3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1
yard = 3 ft; 1 gal = 3.785 L; ?C = 5/9 (?F - 32)
proton charge = +1.6022 x 10-19 C mass = 1.6726 x 10-27 kg
electron charge = -1.6022 x 10-19 C mass = 9.1094 x 10-31 kg
neutron charge = 0 mass = 1.6749 x 10-27 kg
-The distance between two atoms was determined to be 11.4 nm. What is this distance in centimetres?
A) 1.14 x 10-6 cm
B) 11. 4 x 107 cm
C) 114 cm
D) 0.000000114 cm
E) 1.14 x 10-11 cm
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) ?r3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1
yard = 3 ft; 1 gal = 3.785 L; ?C = 5/9 (?F - 32)
proton charge = +1.6022 x 10-19 C mass = 1.6726 x 10-27 kg
electron charge = -1.6022 x 10-19 C mass = 9.1094 x 10-31 kg
neutron charge = 0 mass = 1.6749 x 10-27 kg
-The distance between two atoms was determined to be 11.4 nm. What is this distance in centimetres?
A) 1.14 x 10-6 cm
B) 11. 4 x 107 cm
C) 114 cm
D) 0.000000114 cm
E) 1.14 x 10-11 cm

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
16
Use the following information for Questions
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) ?r3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1
yard = 3 ft; 1 gal = 3.785 L; ?C = 5/9 (?F - 32)
proton charge = +1.6022 x 10-19 C mass = 1.6726 x 10-27 kg
electron charge = -1.6022 x 10-19 C mass = 9.1094 x 10-31 kg
neutron charge = 0 mass = 1.6749 x 10-27 kg
-Four different target shooters fired five shots at targets and their results are shown below. Which of the following statements best describes the precision and accuracy of each "marksman"?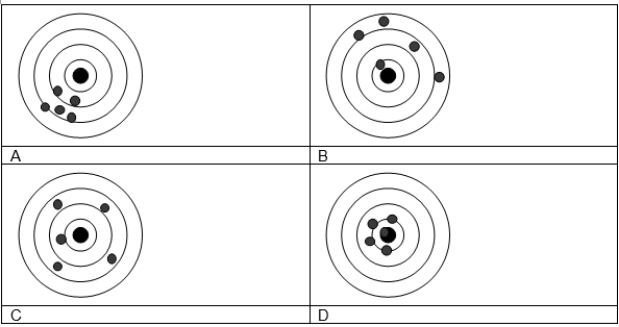
A) A is accurate and precise, C is inaccurate and precise.
B) B is imprecise and inaccurate, D is accurate and imprecise.
C) A and D have similar precision but A is more accurate.
D) C and D have similar accuracy, but D is more precise.
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) ?r3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1
yard = 3 ft; 1 gal = 3.785 L; ?C = 5/9 (?F - 32)
proton charge = +1.6022 x 10-19 C mass = 1.6726 x 10-27 kg
electron charge = -1.6022 x 10-19 C mass = 9.1094 x 10-31 kg
neutron charge = 0 mass = 1.6749 x 10-27 kg
-Four different target shooters fired five shots at targets and their results are shown below. Which of the following statements best describes the precision and accuracy of each "marksman"?

A) A is accurate and precise, C is inaccurate and precise.
B) B is imprecise and inaccurate, D is accurate and imprecise.
C) A and D have similar precision but A is more accurate.
D) C and D have similar accuracy, but D is more precise.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
17
Use the following information for Questions
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) ?r3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1
yard = 3 ft; 1 gal = 3.785 L; ?C = 5/9 (?F - 32)
proton charge = +1.6022 x 10-19 C mass = 1.6726 x 10-27 kg
electron charge = -1.6022 x 10-19 C mass = 9.1094 x 10-31 kg
neutron charge = 0 mass = 1.6749 x 10-27 kg
-What is the answer, with the correct number of significant figures, for the following problem?3.784 g + 56.3 g + 445.55 g =?
A) 505.634 g
B) 505.63 g
C) 505.6 g
D) 505 g
E) 506 g
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) ?r3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1
yard = 3 ft; 1 gal = 3.785 L; ?C = 5/9 (?F - 32)
proton charge = +1.6022 x 10-19 C mass = 1.6726 x 10-27 kg
electron charge = -1.6022 x 10-19 C mass = 9.1094 x 10-31 kg
neutron charge = 0 mass = 1.6749 x 10-27 kg
-What is the answer, with the correct number of significant figures, for the following problem?3.784 g + 56.3 g + 445.55 g =?
A) 505.634 g
B) 505.63 g
C) 505.6 g
D) 505 g
E) 506 g

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
18
Use the following information for Questions
1 mole = 6.022 x1023 particles
-If you had a 50 g sample of C, Al, Fe, Au, and Ti, you would have more atoms of which element?
A) C
B) Al
C) Fe
D) Au
E) Ti
1 mole = 6.022 x1023 particles
-If you had a 50 g sample of C, Al, Fe, Au, and Ti, you would have more atoms of which element?
A) C
B) Al
C) Fe
D) Au
E) Ti

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
19
Use the following information for Questions
1 mole = 6.022 x1023 particles
-If you had a 5.0 g sample of Li, Na, K, Rb, and Cs, you would have more atoms of which element?
A) Li
B) Na
C) K
D) Rb
E) Cs
1 mole = 6.022 x1023 particles
-If you had a 5.0 g sample of Li, Na, K, Rb, and Cs, you would have more atoms of which element?
A) Li
B) Na
C) K
D) Rb
E) Cs

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
20
Use the following information for Questions
1 mole = 6.022 x1023 particles
-How many atoms are present in 52.0 grams of iron?
A) 0.93 atoms
B) 5.61 x 1023
C) 5.61
D) 52
E) 1.20 x 1024
1 mole = 6.022 x1023 particles
-How many atoms are present in 52.0 grams of iron?
A) 0.93 atoms
B) 5.61 x 1023
C) 5.61
D) 52
E) 1.20 x 1024

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
21
Use the following information for Questions
1 mole = 6.022 x1023 particles
-Consider three mole samples of C, Al, Fe, Au, and Ti; which sample will have the greatest mass?
A) C
B) Al
C) Fe
D) Au
E) Ti
1 mole = 6.022 x1023 particles
-Consider three mole samples of C, Al, Fe, Au, and Ti; which sample will have the greatest mass?
A) C
B) Al
C) Fe
D) Au
E) Ti

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
22
How many moles of carbon are present in 20 grams of C3H8?
A) 0.40 moles C
B) 0.80 moles C
C) 1.40 moles C
D) 1.67 moles C
E) 3 moles C
A) 0.40 moles C
B) 0.80 moles C
C) 1.40 moles C
D) 1.67 moles C
E) 3 moles C

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
23
The line structure is shown below for terephthalic acid, a starting material of Kevlar that is used in bullet proof vests: 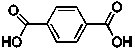 What is the correct molecular formula for this compound?
What is the correct molecular formula for this compound?
A) C6H6 O4
B) C8H2 O4
C) C8H6O4
D) C10H4O4
E) C12H6O4
 What is the correct molecular formula for this compound?
What is the correct molecular formula for this compound?A) C6H6 O4
B) C8H2 O4
C) C8H6O4
D) C10H4O4
E) C12H6O4

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
24
Cumene is an important industrial chemical used in the manufacture of acetone and phenol. The line structure is shown below: 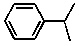 What is the correct molecular formula for cumene?
What is the correct molecular formula for cumene?
A) C8H12
B) C9H11
C) C9H12
D) C10H10
E) C9H16
 What is the correct molecular formula for cumene?
What is the correct molecular formula for cumene?A) C8H12
B) C9H11
C) C9H12
D) C10H10
E) C9H16

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
25
Adipic acid, whose line structure is shown below, is used in the manufacture of nylon used in carpet fibre, among other things. 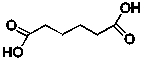 What is the chemical formula for adipic acid?
What is the chemical formula for adipic acid?
A) C4H2O4
B) C6H2O4
C) C6H6O4
D) C6H8O4
E) C6H10O4
 What is the chemical formula for adipic acid?
What is the chemical formula for adipic acid?A) C4H2O4
B) C6H2O4
C) C6H6O4
D) C6H8O4
E) C6H10O4

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
26
Calculate the molar mass of terephthalic acid, whose line structure appears below: 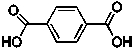
A) 166.13 g/mole
B) 150.13 g/mole
C) 152.12 g/mole
D) 178.14 g/mole
E) 201.14 g/mole

A) 166.13 g/mole
B) 150.13 g/mole
C) 152.12 g/mole
D) 178.14 g/mole
E) 201.14 g/mole

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
27
Calculate the molar mass of ammonium phosphate, (NH4)3PO4.
A) 94.97 g/mole
B) 18.04 g/mole
C) 113.01 g/mole
D) 131.05 g/mole
E) 149.09 g/mole
A) 94.97 g/mole
B) 18.04 g/mole
C) 113.01 g/mole
D) 131.05 g/mole
E) 149.09 g/mole

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
28
Calculate the molar mass of potassium carbonate, K2CO3
A) 39.10 g/mole
B) 60.01 g/mole
C) 99.11 g/mole
D) 138.211 g/mole
E) 177.31 g/mole
A) 39.10 g/mole
B) 60.01 g/mole
C) 99.11 g/mole
D) 138.211 g/mole
E) 177.31 g/mole

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
29
Styrene is used in many plastic products and also in automobile tire rubber. The line structure is shown below: 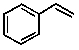 How many hydrogen atoms are contained in 1.05 x 106 kg of styrene?
How many hydrogen atoms are contained in 1.05 x 106 kg of styrene?
A) 4.86 x 1031
B) 6.07 x 1030
C) 1.01 x 107
D) 104
E) 7.71 x 1015
 How many hydrogen atoms are contained in 1.05 x 106 kg of styrene?
How many hydrogen atoms are contained in 1.05 x 106 kg of styrene?A) 4.86 x 1031
B) 6.07 x 1030
C) 1.01 x 107
D) 104
E) 7.71 x 1015

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
30
One litre of air contains about 1 x 10-2 moles of O2.What is the mass of O2 contained in a room 10 m long by 5 m wide by 3 m high?
A) 4.8 g
B) 50g
C) 52 kg
D) 48 kg
E) 1500 g
A) 4.8 g
B) 50g
C) 52 kg
D) 48 kg
E) 1500 g

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
31
How many grams of AgNO3 need to be added to 400 ml of water to make a solution of 0.250 M concentration?
A) 1.70 g
B) 17.0 g
C) 4.25 g
D) 42.5 g
E) 68.0 g
A) 1.70 g
B) 17.0 g
C) 4.25 g
D) 42.5 g
E) 68.0 g

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
32
Nitric acid is a corrosive acid capable of dissolving many metals, with the evolution of brown nitrogen dioxide gas as a byproduct. It is often sold as concentrated nitric acid which has [HNO3] = 16. M. A common concentration used in the laboratory is 6.0 M HNO3. If you need 20. L of 6.0 M HNO3, what volume of concentrated nitric acid is required?
A) 6.7 L
B) 7.5 mL
C) 0.75 L
D) 5.7 L
E) 7.5 L
A) 6.7 L
B) 7.5 mL
C) 0.75 L
D) 5.7 L
E) 7.5 L

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
33
Nitric acid, HNO3, is produced along with water by the reaction of oxygen, O2, with ammonia, NH3, and water, H2O, in a series of steps, with the unbalanced net reaction shown below.
__ NH3 + __ O2 __ HNO3 + __H2O
If the coefficient of NH3 is 1 in the balanced chemical equation of the reaction, what is the coefficient on O2?
A) 2
B) 3
C) 4
D) 5
E) 6
__ NH3 + __ O2 __ HNO3 + __H2O
If the coefficient of NH3 is 1 in the balanced chemical equation of the reaction, what is the coefficient on O2?
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
34
Fertilizer is manufactured from, among other components, phosphoric acid, H3PO4, which, itself is primarily synthesized from fluorapetite, Ca5F(PO4)3 and sulphuric acid, H2SO4.__ H2SO4 + __ Ca5F(PO4)3 __ H3PO4 + __ CaSO4 + __ HFIf the coefficient of H2SO4 is 5 in the balanced chemical equation of the reaction, what is the coefficient of HF?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
35
Two important industrial chemicals, NaOH and chlorine, are produced from solutions of sodium chloride by the following reaction:
__ NaCl + __ H2O __ NaOH + __ Cl2 + __ H2
If the coefficient of H2O is 2 in the balanced chemical equation of reaction, what is the coefficient of NaOH?
A) 1
B) 2
C) 3
D) 4
E) 5
__ NaCl + __ H2O __ NaOH + __ Cl2 + __ H2
If the coefficient of H2O is 2 in the balanced chemical equation of reaction, what is the coefficient of NaOH?
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
36
A 1.345 g sample of solid residue from a hazardous waste dump is being analyzed for barium. The sample is dissolved and then sodium sulphate is added. The insoluble barium sulphate is dried, and a total of 73.8 mg of BaSO4 is collected. What percent of the sample is barium?
A) 0.323 % Ba
B) 2.33 % Ba
C) 3.23 % Ba
D) 4.02 % Ba
E) 5.23 % Ba
A) 0.323 % Ba
B) 2.33 % Ba
C) 3.23 % Ba
D) 4.02 % Ba
E) 5.23 % Ba

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
37
Sodium sulphate is used in detergents and can be synthesized by the following unbalanced reaction:
NaCl + H2SO4 Na2SO4 + HCl
How much sulphuric acid (in kg) would be required to consume 5.0 x 103 kg of NaCl?
A) 4.3 x 106 kg
B) 4.2 x103 kg
C) 4.4 x 104 kg
D) 5.0 x 106 kg
E) 8.7 x 104 kg
NaCl + H2SO4 Na2SO4 + HCl
How much sulphuric acid (in kg) would be required to consume 5.0 x 103 kg of NaCl?
A) 4.3 x 106 kg
B) 4.2 x103 kg
C) 4.4 x 104 kg
D) 5.0 x 106 kg
E) 8.7 x 104 kg

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
38
Aluminum sulphate is used in sand paper, among other uses. How many g of aluminum oxide dihydrate will be needed to produce 500 g of aluminum sulphate by the (unbalanced) reaction below?
Al2O3 •2H2O + H2SO4 Al2(SO4)3 + H2O
A) 1.46
B) 40.0
C) 202
D) 342
E) 500
Al2O3 •2H2O + H2SO4 Al2(SO4)3 + H2O
A) 1.46
B) 40.0
C) 202
D) 342
E) 500

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
39
The industrial synthesis of methanol, CH3OH, is catalyzed by metal oxides. Billions of kg are produced annually for use in polymers and fuel additives.
CO + 2 H2 CH3OH
How many kg of hydrogen will be required to produce 1.0 x 105 kg of methanol?
A) 3.1 x 106
B) 6.2 x 103
C) 6.2 x 106
D) 1.3 x 107
E) 1.3 x 104
CO + 2 H2 CH3OH
How many kg of hydrogen will be required to produce 1.0 x 105 kg of methanol?
A) 3.1 x 106
B) 6.2 x 103
C) 6.2 x 106
D) 1.3 x 107
E) 1.3 x 104

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
40
A way of generating dry O2 in the lab is to heat potassium chlorate. If you determined that 25 grams of O2 are needed, how much potassium chlorate do you need to start with? 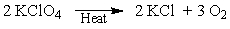
A) 64 grams
B) 96 grams
C) 17 grams
D) 25 grams
E) 128 grams
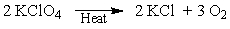
A) 64 grams
B) 96 grams
C) 17 grams
D) 25 grams
E) 128 grams

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
41
Urea is used as a source of nitrogen in fertilizer. The second step of the synthesis is heating the intermediate ammonium carbamate to produce urea shown in the equation below:
NH2CO2 NH4 O)NH2 + H2O
The reaction proceeds in 60% yield, on average, and the product is mixed with unreacted starting materials, which are readily separated from the product. They can then be recycled and undergo the process again. If one starts with 1 kg of ammonium carbamate, how many times will it have to be cycled through this reaction to give at least a 90% yield?
A) 2
B) 3
C) 4
D) 5
E) infinite number of times
NH2CO2 NH4 O)NH2 + H2O
The reaction proceeds in 60% yield, on average, and the product is mixed with unreacted starting materials, which are readily separated from the product. They can then be recycled and undergo the process again. If one starts with 1 kg of ammonium carbamate, how many times will it have to be cycled through this reaction to give at least a 90% yield?
A) 2
B) 3
C) 4
D) 5
E) infinite number of times

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
42
How many kg of ethylene oxide is needed to produce 1.05 x 104 kg of ethylene glycol by the following reaction that proceeds in 95% yield? 
A) 1.69 x 105
B) 1.78 x 105
C) 5.23 x 103
D) 7.45 x 103
E) 7.84 x103

A) 1.69 x 105
B) 1.78 x 105
C) 5.23 x 103
D) 7.45 x 103
E) 7.84 x103

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
43
A way of generating dry O2 in the lab is to heat potassium chlorate. If you determined that 25 grams of O2 are needed, how much potassium chlorate do you need to start with if there is only a 60% yield? 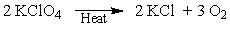
A) 64 grams
B) 96 grams
C) 17 grams
D) 25 grams
E) 106 grams
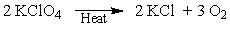
A) 64 grams
B) 96 grams
C) 17 grams
D) 25 grams
E) 106 grams

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
44
Hydrazine (N2H4) is made by the reaction of ammonia (NH3) with hypochlorite (OCl-). Given the following equation 420 kg of ammonia is reacted with excess hypochlorite to generate 315 kg of hydrazine. What is the percent yield for this unbalanced reaction?
NH3 + OCl- N2H4 + Cl- + H2O
A) 75.0 %
B) 79.7 %
C) 150 %
D) 20.3 %
E) 66.7 %
NH3 + OCl- N2H4 + Cl- + H2O
A) 75.0 %
B) 79.7 %
C) 150 %
D) 20.3 %
E) 66.7 %

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
45
Adiponitrile is produced by the reaction of HCN and butadiene: 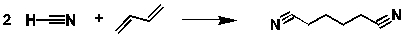 If 450 kg of butadiene and 400 kg of HCN were to react to completion, what is the maximum amount of adiponitrile that would be produced?
If 450 kg of butadiene and 400 kg of HCN were to react to completion, what is the maximum amount of adiponitrile that would be produced?
A) 450 kg
B) 8.32 x 103 moles
C) 1.48 x 103 moles
D) 800 kg
E) 900 kg
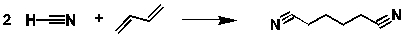 If 450 kg of butadiene and 400 kg of HCN were to react to completion, what is the maximum amount of adiponitrile that would be produced?
If 450 kg of butadiene and 400 kg of HCN were to react to completion, what is the maximum amount of adiponitrile that would be produced?A) 450 kg
B) 8.32 x 103 moles
C) 1.48 x 103 moles
D) 800 kg
E) 900 kg

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
46
Aluminum sulphate is used in finishing paper, among other uses. If 500 kg of aluminum oxide dihydrate react completely with 1500 kg of 90% by mass aqueous H2SO4, how many kg of aluminum sulphate can be produced?
Al2O3 •2H2O + 3H2SO4 Al2(SO4)3 + 5H2O
A) 1.24 x 103 kg
B) 1.35 x 103 kg
C) 1.57 x103 kg
D) 3.62 x103 kg
E) 4.59 x103 kg
Al2O3 •2H2O + 3H2SO4 Al2(SO4)3 + 5H2O
A) 1.24 x 103 kg
B) 1.35 x 103 kg
C) 1.57 x103 kg
D) 3.62 x103 kg
E) 4.59 x103 kg

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
47
Thermite is a reaction that was once used to weld railroads together as shown below. A 48.0 g sample of Al(s) was reacted with 99.0 g of Fe2O3 according to the equation below. What could be the theoretical yield of Fe(s) in grams?
2 Al(s) + Fe2O3(s) Al2O3(s) + 2 Fe(s)
A) 52.5 g
B) 99.3 g
C) 69.2 g
D) 48.0 g
E) 175 g
2 Al(s) + Fe2O3(s) Al2O3(s) + 2 Fe(s)
A) 52.5 g
B) 99.3 g
C) 69.2 g
D) 48.0 g
E) 175 g

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
48
Potassium permanganate reacts with hydrochloric acid to give chlorine gas according to unbalanced chemical reaction shown below. If 34.2 g of KMnO4 were mixed with 0.990 moles of HCl, how many moles of chlorine gas could be produced?
KMnO4 + HCl MnCl2 + KCl + Cl2(g) + H2O
A) 0.541 moles
B) 0.850 moles
C) 0.309 moles
D) 0.989 moles
E) 0.354 moles
KMnO4 + HCl MnCl2 + KCl + Cl2(g) + H2O
A) 0.541 moles
B) 0.850 moles
C) 0.309 moles
D) 0.989 moles
E) 0.354 moles

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
49
What are the symbols for the following elements?sulphur, fluorine, titanium, potassium, zinc

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
50
What are the names of the elements with the following symbols?
Cr, Na, Cl, Be, He
Cr, Na, Cl, Be, He

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
51
What are the names of the elements with the following symbols?
Mn, Mg, Md, Rn, Ra
Mn, Mg, Md, Rn, Ra

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
52
Write formulas of the compounds whose molecular pictures are shown below (unlabelled atoms are carbon). 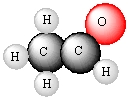
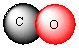
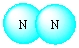
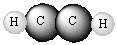







Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
53
Determine the formula of a compound whose molecules each contain two atoms of carbon, two atoms of oxygen and six atoms of hydrogen.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
54
Name three macroscopic properties of orange juice.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
55
Plastics are one class of a type of very large molecules called polymers. They are built from smaller building blocks, such as propene. Propene has three carbon atoms and six hydrogen atoms. What is its chemical formula?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
56
What are two microscopic properties of a substance?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
57
Use the following information for Questions
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-The drive to San Francisco from Los Angeles along the coastal route is 450 miles. What is the distance from Los Angeles to San Francisco expressed in SI units using scientific notation?
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-The drive to San Francisco from Los Angeles along the coastal route is 450 miles. What is the distance from Los Angeles to San Francisco expressed in SI units using scientific notation?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
58
Use the following information for Questions
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-What is the volume in SI units of a swimming pool that is 4.5 feet deep by 5 yards wide and 25 yards long?
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-What is the volume in SI units of a swimming pool that is 4.5 feet deep by 5 yards wide and 25 yards long?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
59
Use the following information for Questions
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-You fill your car up in Jamaica and it takes 42.3 L of gasoline. How many gallons is this amount?
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-You fill your car up in Jamaica and it takes 42.3 L of gasoline. How many gallons is this amount?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
60
Use the following information for Questions
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-Gold has a density of 19.282 g/cm3. What is the mass (in kg) of a sphere of solid gold with a radius of 6.0 cm? (The volume of a sphere is V = EQ \F(4,3) πr3 where r is the radius.)
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-Gold has a density of 19.282 g/cm3. What is the mass (in kg) of a sphere of solid gold with a radius of 6.0 cm? (The volume of a sphere is V = EQ \F(4,3) πr3 where r is the radius.)

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
61
Use the following information for Questions
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-What is the temperature of liquid nitrogen in ˚F, if the nitrogen is currently at -195.8 ˚C?
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-What is the temperature of liquid nitrogen in ˚F, if the nitrogen is currently at -195.8 ˚C?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
62
Use the following information for Questions
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-A flask with a mass of 160.342 g is filled with 22° C water. The mass of the flask filled with water is found to be 310.5 g. Given that the density of water at 22°C is 0.99780 g/cm3, what is the volume of the flask?
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-A flask with a mass of 160.342 g is filled with 22° C water. The mass of the flask filled with water is found to be 310.5 g. Given that the density of water at 22°C is 0.99780 g/cm3, what is the volume of the flask?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
63
Use the following information for Questions
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-Fill in the missing quantity: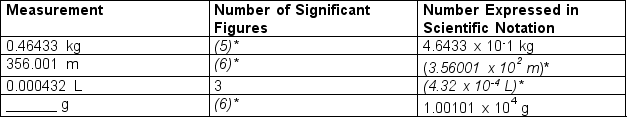
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-Fill in the missing quantity:


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
64
Use the following information for Questions
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-When you fill up your car in a country in Europe, your credit card is charged $25.45 for 32.3 L of gasoline. What is the price of gasoline in dollars per gallon?
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-When you fill up your car in a country in Europe, your credit card is charged $25.45 for 32.3 L of gasoline. What is the price of gasoline in dollars per gallon?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
65
Use the following information for Questions
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-Rhodium metal recently was selling for $105.15 per gram. What is the price for a pound of rhodium metal in dollars?
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-Rhodium metal recently was selling for $105.15 per gram. What is the price for a pound of rhodium metal in dollars?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
66
Use the following information for Questions
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-It has been said that 3.0 kg of plutonium is enough to make an atomic bomb. If you were working security at an airport, just how large of a metallic object would you be looking for? That is, determine the diameter (in cm) of a sphere of plutonium that has a mass of 3.0 kg. (the density of plutonium is 19.84 g/cm3)
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-It has been said that 3.0 kg of plutonium is enough to make an atomic bomb. If you were working security at an airport, just how large of a metallic object would you be looking for? That is, determine the diameter (in cm) of a sphere of plutonium that has a mass of 3.0 kg. (the density of plutonium is 19.84 g/cm3)

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
67
Use the following information for Questions
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-You are asked to administer 4.9 g of an injectable solution which contains 0.23 % of a lifesaving drug by mass. The syringe has graduations of 0.1 mL. If the density of the solution is 1.13 g/mL, how many mL should you inject?
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-You are asked to administer 4.9 g of an injectable solution which contains 0.23 % of a lifesaving drug by mass. The syringe has graduations of 0.1 mL. If the density of the solution is 1.13 g/mL, how many mL should you inject?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
68
Use the following information for Questions
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-Ethyl ether has a density of 0.7138 g/ml. How many litres will be needed to obtain 500 grams of ethyl ether for a reaction?
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-Ethyl ether has a density of 0.7138 g/ml. How many litres will be needed to obtain 500 grams of ethyl ether for a reaction?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
69
Use the following information for Questions
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-The density of mercury is 13.6 g/ml and the density of water is 1.00 g/ml. How many meters high would a column of water need to be, to be equal in mass to a column of mercury 750 mm high? (assume the columns of liquid have the same diameter)
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-The density of mercury is 13.6 g/ml and the density of water is 1.00 g/ml. How many meters high would a column of water need to be, to be equal in mass to a column of mercury 750 mm high? (assume the columns of liquid have the same diameter)

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
70
Use the following information for Questions
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-What is the difference in mass between 1.000 x 1015 protons and the same number of neutrons? What is the electrical charge (in coulombs) carried by this number of protons?
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-What is the difference in mass between 1.000 x 1015 protons and the same number of neutrons? What is the electrical charge (in coulombs) carried by this number of protons?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
71
Use the following information for Questions
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-What is the mass of 1.000 x 1015 electrons? What is the electrical charge (in coulombs) carried by this number of electrons?
3.785 L = 1 gallon; 2.2 lbs = 1 kg; V = EQ \F(4,3) πr3; 1 in = 2.54 cm; 1 mile = 1.609 km; 12 in = 1 ft; 1 yard = 3 ft; 1 gal = 3.785 L; ˚C = 5/9 (˚F - 32)

-What is the mass of 1.000 x 1015 electrons? What is the electrical charge (in coulombs) carried by this number of electrons?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
72
Use the following information for Questions .
1 mole = 6.022 x1023 particles
-Which would have a greater mass: 1.25 mole sample of lead or 11.25 mole sample of aluminum?
1 mole = 6.022 x1023 particles
-Which would have a greater mass: 1.25 mole sample of lead or 11.25 mole sample of aluminum?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
73
Use the following information for Questions .
1 mole = 6.022 x1023 particles
-Which would have a greater mass: a 1.75 mole sample of tin or a 5.25 mole sample of aluminum?
1 mole = 6.022 x1023 particles
-Which would have a greater mass: a 1.75 mole sample of tin or a 5.25 mole sample of aluminum?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
74
Use the following information for Questions .
1 mole = 6.022 x1023 particles
-Which would have a greater mass: a 2.37 mole sample of potassium or a 3.72 mole sample of magnesium?
1 mole = 6.022 x1023 particles
-Which would have a greater mass: a 2.37 mole sample of potassium or a 3.72 mole sample of magnesium?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
75
Use the following information for Questions .
1 mole = 6.022 x1023 particles
-Naturally occurring platinum has several different isotopes, 190Pt (0.0127%; 189.960 amu); 192Pt (0.78 %; 191.9614 amu); 194Pt (32.9%; 193.9628 amu), 195Pt (33.8 %; 194.9648 amu); 196Pt (25.3 %; 195.9650 amu) and 198Pt (7.21 %; 197.9675 amu). What is the molar mass of naturally occurring platinum?
1 mole = 6.022 x1023 particles
-Naturally occurring platinum has several different isotopes, 190Pt (0.0127%; 189.960 amu); 192Pt (0.78 %; 191.9614 amu); 194Pt (32.9%; 193.9628 amu), 195Pt (33.8 %; 194.9648 amu); 196Pt (25.3 %; 195.9650 amu) and 198Pt (7.21 %; 197.9675 amu). What is the molar mass of naturally occurring platinum?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
76
Use the following information for Questions .
1 mole = 6.022 x1023 particles
-The density of a particular 300 cm3 graphite sample is 2.16 g cm-3; how many atoms are contained in the sample?
1 mole = 6.022 x1023 particles
-The density of a particular 300 cm3 graphite sample is 2.16 g cm-3; how many atoms are contained in the sample?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
77
Use the following information for Questions .
1 mole = 6.022 x1023 particles
-What volume is occupied by an iron sample containing 7.2 x 1025 atoms if the density of the sample is 7.87 g cm3?
1 mole = 6.022 x1023 particles
-What volume is occupied by an iron sample containing 7.2 x 1025 atoms if the density of the sample is 7.87 g cm3?

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
78
Calculate the number of moles of carbon atoms and moles of sulphur atoms in 50.0 g of CS2.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
79
Draw a ball and stick model of 1,6-hexanediamine, a starting material for nylon. The line structure is shown below: 


Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
80
Determine the molecular formula and draw the line structure for 2-bromobutane.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck


