Deck 12: Metabolism and Bioenergetics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/40
Play
Full screen (f)
Deck 12: Metabolism and Bioenergetics
1
Plants are considered _____.
A) chemoautotrophs
B) photoautotrophs
C) chemoheterotrophs
D) photoheterotrophs
E) none of the above
A) chemoautotrophs
B) photoautotrophs
C) chemoheterotrophs
D) photoheterotrophs
E) none of the above
photoautotrophs
2
Digestion of food by mammals converts _____ into _____ which can be absorbed from the intestines.
A) proteins; dipeptides and tripeptides
B) polysaccharides; monosaccharides and disaccharides
C) cholesterol; acetyl CoA
D) nucleic acids; polynucleotides
E) triacylglycerols; fatty acids
A) proteins; dipeptides and tripeptides
B) polysaccharides; monosaccharides and disaccharides
C) cholesterol; acetyl CoA
D) nucleic acids; polynucleotides
E) triacylglycerols; fatty acids
triacylglycerols; fatty acids
3
Digestion of food utilizes enzymes that catalyze _____ reactions.
A) hydrolysis
B) reduction
C) condensation
D) oxidative
E) group transfer
A) hydrolysis
B) reduction
C) condensation
D) oxidative
E) group transfer
hydrolysis
4
Which of the following is used to move lipids around the bloodstream?
A) micelles
B) liposomes
C) liposuctions
D) lipoproteins
E) cholesterol esters
A) micelles
B) liposomes
C) liposuctions
D) lipoproteins
E) cholesterol esters

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
5
The bulk of triacylglycerols in the human body are stored in _____.
A) liver cells
B) adipocytes
C) muscle cells
D) nerve cells
E) lipoproteins
A) liver cells
B) adipocytes
C) muscle cells
D) nerve cells
E) lipoproteins

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
6
The _____ stores glucose as glycogen and converts excess glucose to _____.
A) muscle; amino acids
B) adipose tissue; fatty acids
C) liver; fatty acids
D) brain; energy
E) none of the above
A) muscle; amino acids
B) adipose tissue; fatty acids
C) liver; fatty acids
D) brain; energy
E) none of the above

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
7
Amino acids can be converted to _____.
A) carbohydrates
B) fatty acids
C) nucleotides
D) peptides
E) all of the above
A) carbohydrates
B) fatty acids
C) nucleotides
D) peptides
E) all of the above

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is mobilized for energy use by a phosphorolysis reaction, not a hydrolysis?
A) glycogen
B) protein
C) triacylglycerols
D) polynucleotides
E) cholesterol esters
A) glycogen
B) protein
C) triacylglycerols
D) polynucleotides
E) cholesterol esters

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
9
Degradation of proteins within a cell can occur within the _____ or by use of _____.
A) endoplasmic reticulum; protease enzymes chymotrypsin and trypsin
B) Golgi apparatus; protease enzymes chymotrypsin and trypsin
C) endoplasmic reticulum; a proteasome
D) lysosome; a proteasome
E) mitochondria; ubiquitin
A) endoplasmic reticulum; protease enzymes chymotrypsin and trypsin
B) Golgi apparatus; protease enzymes chymotrypsin and trypsin
C) endoplasmic reticulum; a proteasome
D) lysosome; a proteasome
E) mitochondria; ubiquitin

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
10
The _____-terminus of ubiquitin is linked to a _____ residue of proteins to be degraded.
A) N; Asp
B) N; Glu
C) C; Lys
D) C; Ser
E) C; Tyr
A) N; Asp
B) N; Glu
C) C; Lys
D) C; Ser
E) C; Tyr

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following molecules is involved with the oxidation of glucose, synthesis of fatty acids and oxidation of fatty acids?
A) pyruvate
B) acetyl-CoA
C) alanine
D) oxaloacetate
E) glyceraldehyde-3-phosphate
A) pyruvate
B) acetyl-CoA
C) alanine
D) oxaloacetate
E) glyceraldehyde-3-phosphate

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is more highly oxidized than acetaldehyde?
A) ethane
B) ethanol
C) ethylene
D) ethylene glycol
E) acetic acid
A) ethane
B) ethanol
C) ethylene
D) ethylene glycol
E) acetic acid

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
13
The oxidized form of NADH is _____.
A) NADH+
B) NAD+
C) NADH
D) NADH2
E) none of the above
A) NADH+
B) NAD+
C) NADH
D) NADH2
E) none of the above

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following molecules is in the most reduced state?
A) methane
B) formaldehyde
C) formic acid
D) methanol
E) carbon dioxide
A) methane
B) formaldehyde
C) formic acid
D) methanol
E) carbon dioxide

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
15
The conversion of a carbohydrate into CO2 is a(n) _____ process; the conversion of CO2 into a carbohydrate is a(n) _____ process.
A) reductive; oxidative
B) endergonic; exergonic
C) exergonic; endergonic
D) oxidative; exergonic
E) endergonic; reductive
A) reductive; oxidative
B) endergonic; exergonic
C) exergonic; endergonic
D) oxidative; exergonic
E) endergonic; reductive

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is a lipid-soluble electron carrier in its reduced state?
A) NADPH
B) NADH
C) NAD+
D) ubiquinol
E) ubiquinone
A) NADPH
B) NADH
C) NAD+
D) ubiquinol
E) ubiquinone

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is correct regarding metabolic pathways?
A) most pathways are isolated from other pathways
B) the activity of most pathways is not regulated
C) all cells within a multi-cellular organism contain the same pathways
D) anabolic pathways never occur at the same time as catabolic pathways
E) none of the above are correct
A) most pathways are isolated from other pathways
B) the activity of most pathways is not regulated
C) all cells within a multi-cellular organism contain the same pathways
D) anabolic pathways never occur at the same time as catabolic pathways
E) none of the above are correct

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is correctly described?
A) proteome: the complete set of proteins present in a cell at a given time
B) metabolome: the complete set of metabolic enzymes active in a cell at a given time
C) transcriptome: the complete set of proteins being synthesized at a given time
D) genome: the complete set of genes that are expressed at a given time
E) none of the above
A) proteome: the complete set of proteins present in a cell at a given time
B) metabolome: the complete set of metabolic enzymes active in a cell at a given time
C) transcriptome: the complete set of proteins being synthesized at a given time
D) genome: the complete set of genes that are expressed at a given time
E) none of the above

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is an essential amino acid?
A) Ala
B) Val
C) Cys
D) Tyr
E) Gly
A) Ala
B) Val
C) Cys
D) Tyr
E) Gly

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following vitamins is correctly paired with its biochemical function?
A) folic acid: carboxylation reactions
B) biotin: decarboxylation reactions
C) riboflavin: acyl transfer reactions
D) pyridoxine: amino-group transfer reactions
E) pantothenic acid: redox reactions
A) folic acid: carboxylation reactions
B) biotin: decarboxylation reactions
C) riboflavin: acyl transfer reactions
D) pyridoxine: amino-group transfer reactions
E) pantothenic acid: redox reactions

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following vitamins is correctly paired with the disease that is caused by its deficiency?
A) biotin: scurvy
B) pantothenic acid: beriberi
C) nicotinamide: pellagra
D) thiamine: anemia
E) folic acid: rickets
A) biotin: scurvy
B) pantothenic acid: beriberi
C) nicotinamide: pellagra
D) thiamine: anemia
E) folic acid: rickets

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
22
When a reaction is at equilibrium, the G is equal to _____.
A) 1
B) 0
C) -1
D) G°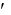
E) none of the above
A) 1
B) 0
C) -1
D) G°
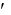
E) none of the above

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
23
If the G° 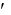 is known, how can Keq be calculated?
is known, how can Keq be calculated?
A) Keq = log e( G° 11ee7c68_2bc0_26ba_ae82_e338791695a2_TBW1044_11 /RT)
B) Keq = e( G° 11ee7c68_2bc0_26ba_ae82_e338791695a2_TBW1044_11 /T S)
C) Keq = RT ln G° 11ee7c68_2bc0_26ba_ae82_e338791695a2_TBW1044_11
D) Keq = e-( G° 11ee7c68_2bc0_26ba_ae82_e338791695a2_TBW1044_11 /RT)
E) Keq = ln ( G° 11ee7c68_2bc0_26ba_ae82_e338791695a2_TBW1044_11 /T S)
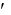 is known, how can Keq be calculated?
is known, how can Keq be calculated?A) Keq = log e( G° 11ee7c68_2bc0_26ba_ae82_e338791695a2_TBW1044_11 /RT)
B) Keq = e( G° 11ee7c68_2bc0_26ba_ae82_e338791695a2_TBW1044_11 /T S)
C) Keq = RT ln G° 11ee7c68_2bc0_26ba_ae82_e338791695a2_TBW1044_11
D) Keq = e-( G° 11ee7c68_2bc0_26ba_ae82_e338791695a2_TBW1044_11 /RT)
E) Keq = ln ( G° 11ee7c68_2bc0_26ba_ae82_e338791695a2_TBW1044_11 /T S)

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
24
For the following reaction, calculate the Keq at 25°C.
Succinyl-CoA + acetoacetate acetoacetyl-CoA + succinate G°
acetoacetyl-CoA + succinate G° 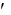 = -1.25 kJ/mol
= -1.25 kJ/mol
A) 0.602
B) 1.00
C) 1.66
D) 3.21
E) 4.22c*c 102
Succinyl-CoA + acetoacetate
 acetoacetyl-CoA + succinate G°
acetoacetyl-CoA + succinate G° 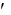 = -1.25 kJ/mol
= -1.25 kJ/molA) 0.602
B) 1.00
C) 1.66
D) 3.21
E) 4.22c*c 102

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
25
For the following reaction, calculate the G° at 37°C.
Glucose-6-phosphate fructose-6-phosphate Keq = 0.517
fructose-6-phosphate Keq = 0.517
A) -2.87 kJ/mol
B) -1.70 kJ/mol
C) 0.203 kJ/mol
D) -0.738 kJ/mol
E) 1.70 kJ/mol
Glucose-6-phosphate
 fructose-6-phosphate Keq = 0.517
fructose-6-phosphate Keq = 0.517A) -2.87 kJ/mol
B) -1.70 kJ/mol
C) 0.203 kJ/mol
D) -0.738 kJ/mol
E) 1.70 kJ/mol

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
26
For the following reaction, calculate the G at 37°C, given concentrations for glucose-1-phosphate of 25 mM and glucose-6-phosphate of 1 mM.
Glucose-1-phosphate glucose-6-phosphate G°
glucose-6-phosphate G° 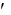 = -7.1 kJ/mol
= -7.1 kJ/mol
A) -15.4 kJ/mol
B) -8.1 kJ/mol
C) -6.1 kJ/mol
D) 1.2 kJ/mol
E) none of the above
Glucose-1-phosphate
 glucose-6-phosphate G°
glucose-6-phosphate G° 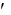 = -7.1 kJ/mol
= -7.1 kJ/molA) -15.4 kJ/mol
B) -8.1 kJ/mol
C) -6.1 kJ/mol
D) 1.2 kJ/mol
E) none of the above

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
27
What is the intracellular glucose concentration if the G for the following reaction is -20.1 kJ/mol at 37°C and concentrations for glucose-6-phosphate and phosphate are both 1 mM?
Glucose-6-phosphate glucose + Pi G°
glucose + Pi G° 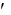 = -13.8 kJ/mol
= -13.8 kJ/mol
A) 1.9 M
B) 87 M
C) 1.9 mM
D) 27 mM
E) 87 mM
Glucose-6-phosphate
 glucose + Pi G°
glucose + Pi G° 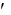 = -13.8 kJ/mol
= -13.8 kJ/molA) 1.9 M
B) 87 M
C) 1.9 mM
D) 27 mM
E) 87 mM

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following describes the bonding of the three phosphates to adenosine in ATP? How many phosphoanhydride bonds are found in ATP?
A) one phosphoanhydride bond, two low energy phosphate esters
B) two phosphoanhydride bonds, one low energy phosphate ester
C) three phosphoanhydride bonds, one low energy phosphate ester
D) three phosphoanhydride bonds
E) none of the above
A) one phosphoanhydride bond, two low energy phosphate esters
B) two phosphoanhydride bonds, one low energy phosphate ester
C) three phosphoanhydride bonds, one low energy phosphate ester
D) three phosphoanhydride bonds
E) none of the above

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
29
If the following reactions were coupled, what would be the overall G° 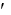
Glucose + Pi glucose-6-phosphate G° 11ee7c69_05ae_405c_ae82_f7bc848d8fc6_TBW1044_11 = 13.8 kJ/mol
glucose-6-phosphate G° 11ee7c69_05ae_405c_ae82_f7bc848d8fc6_TBW1044_11 = 13.8 kJ/mol
ATP + H2O ADP + Pi G° 11ee7c69_05ae_405c_ae82_f7bc848d8fc6_TBW1044_11 = -30.5 kJ/mol
ADP + Pi G° 11ee7c69_05ae_405c_ae82_f7bc848d8fc6_TBW1044_11 = -30.5 kJ/mol
A) 44.3 kJ/mol
B) 16.7 kJ/mol
C) 0 kJ/mol
D) -16.7 kJ/mol
E) -44.3 kJ/mol
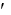
Glucose + Pi
 glucose-6-phosphate G° 11ee7c69_05ae_405c_ae82_f7bc848d8fc6_TBW1044_11 = 13.8 kJ/mol
glucose-6-phosphate G° 11ee7c69_05ae_405c_ae82_f7bc848d8fc6_TBW1044_11 = 13.8 kJ/molATP + H2O
 ADP + Pi G° 11ee7c69_05ae_405c_ae82_f7bc848d8fc6_TBW1044_11 = -30.5 kJ/mol
ADP + Pi G° 11ee7c69_05ae_405c_ae82_f7bc848d8fc6_TBW1044_11 = -30.5 kJ/molA) 44.3 kJ/mol
B) 16.7 kJ/mol
C) 0 kJ/mol
D) -16.7 kJ/mol
E) -44.3 kJ/mol

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following factors contributes to the highly exergonic nature of ATP hydrolysis?
A) removal of phosphate from the cytoplasm
B) addition of water to the hydrophilic ATP molecule
C) decrease in negative-ion repulsion in ATP
D) low energy of activation for the hydrolysis
E) none of the above
A) removal of phosphate from the cytoplasm
B) addition of water to the hydrophilic ATP molecule
C) decrease in negative-ion repulsion in ATP
D) low energy of activation for the hydrolysis
E) none of the above

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following has the most spontaneous hydrolysis?
A) 1,3-bisphosphoglycerate
B) phosphocreatine
C) glucose-1-phosphate
D) pyrophosphate
E) phosphoenolpyruvate
A) 1,3-bisphosphoglycerate
B) phosphocreatine
C) glucose-1-phosphate
D) pyrophosphate
E) phosphoenolpyruvate

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
32
In highly active muscle, _____ is used to regenerate ATP.
A) phosphocreatine
B) 1,3-bisphosphoglycerate
C) pyrophosphate
D) phosphoenolpyruvate
E) acetyl-CoA
A) phosphocreatine
B) 1,3-bisphosphoglycerate
C) pyrophosphate
D) phosphoenolpyruvate
E) acetyl-CoA

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
33
The G° 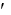 for the hydrolysis of acetyl CoA is most similar to the G° 11ee7c69_05ae_405c_ae82_f7bc848d8fc6_TBW1044_11 for the hydrolysis of _____.
for the hydrolysis of acetyl CoA is most similar to the G° 11ee7c69_05ae_405c_ae82_f7bc848d8fc6_TBW1044_11 for the hydrolysis of _____.
A) 1,3-bisphosphoglycerate
B) ATP
C) glucose-1-phosphate
D) glucose-6-phosphate
E) phosphoenolpyruvate
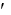 for the hydrolysis of acetyl CoA is most similar to the G° 11ee7c69_05ae_405c_ae82_f7bc848d8fc6_TBW1044_11 for the hydrolysis of _____.
for the hydrolysis of acetyl CoA is most similar to the G° 11ee7c69_05ae_405c_ae82_f7bc848d8fc6_TBW1044_11 for the hydrolysis of _____.A) 1,3-bisphosphoglycerate
B) ATP
C) glucose-1-phosphate
D) glucose-6-phosphate
E) phosphoenolpyruvate

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
34
Metabolomics is the study of the metabolic activity of a cell or tissue by identifying and quantifying all of its metabolites.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
35
The amino acid ___ is considered essential in humans while ___ is considered non-essential.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
36
The reaction below is an example of _________________. 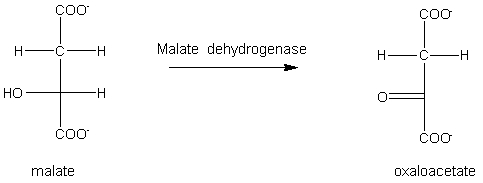
A) dehydration
B) hydration
C) oxidation
D) reduction
E) none of the above

A) dehydration
B) hydration
C) oxidation
D) reduction
E) none of the above

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
37
Eukaryotes evolved and developed extensive intracellular membraines enclosed in compartments called _______.
A) lysosomes
B) organelles
C) peroxisomes
D) vacuoles
A) lysosomes
B) organelles
C) peroxisomes
D) vacuoles

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
38
Consider the reaction A + B « C + D. After reaching equilibrium at 25°C, the following concentrations of reactants and products were measured: [A] = 10 µM, [B] = 15 µM, [C] = 10 µM, [D] = 10 µM. Calculate G°![<strong>Consider the reaction A + B « C + D. After reaching equilibrium at 25°C, the following concentrations of reactants and products were measured: [A] = 10 µM, [B] = 15 µM, [C] = 10 µM, [D] = 10 µM. Calculate \Delta G° for this reaction.</strong> A) 1,000 J/mol B) 10 kJ/mol C) 1 J/mol D) insufficient data to determine answer E) none of the above](https://storage.examlex.com/TBW1044/11ee7c69_6fa3_30bd_ae82_e78d2f88d356_TBW1044_11.jpg) for this reaction.
for this reaction.
A) 1,000 J/mol
B) 10 kJ/mol
C) 1 J/mol
D) insufficient data to determine answer
E) none of the above
![<strong>Consider the reaction A + B « C + D. After reaching equilibrium at 25°C, the following concentrations of reactants and products were measured: [A] = 10 µM, [B] = 15 µM, [C] = 10 µM, [D] = 10 µM. Calculate \Delta G° for this reaction.</strong> A) 1,000 J/mol B) 10 kJ/mol C) 1 J/mol D) insufficient data to determine answer E) none of the above](https://storage.examlex.com/TBW1044/11ee7c69_6fa3_30bd_ae82_e78d2f88d356_TBW1044_11.jpg) for this reaction.
for this reaction.A) 1,000 J/mol
B) 10 kJ/mol
C) 1 J/mol
D) insufficient data to determine answer
E) none of the above

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
39
Phosphoglucomutase catalyzes the reaction in which a phosphate group is transferred from the C-1 of glucose to the C-6 of glucose (G1P G6P). A student incubates a 0.2 M solution of glucose-1-phosphate overnight with a small amount of the enzyme. At equilibrium the concentration of glucose-1-phosphate is 9.0 × 10-3 M and the concentration of glucose-6-phosphate is 19.1 × 10-2 M.
Calculate the equilibrium constant (Keq) and the standard state free energy ( G°') for this reaction at 25°C.
Calculate the equilibrium constant (Keq) and the standard state free energy ( G°') for this reaction at 25°C.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
40
Acetylcholinesterase catalyzes the reaction in which acetylcholine is hydrolyzed into acetate and choline. This is a reversible reaction. A student incubates a 1 M solution of acetylcholine for two hours with 100uL of acetylcholinesterase. At equilibrium the concentration of acetylcholine is 3.0 × 10 mM and the concentration of acetate and choline are 5.2 × 10-3 M and 7.4 x 10-2 M, respectively.
Calculate the equilibrium constant (Keq) and the standard state free energy ( G°') for this reaction at 17°C.
Calculate the equilibrium constant (Keq) and the standard state free energy ( G°') for this reaction at 17°C.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck


