Deck 7: Enzyme Kinetics and Inhibition
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/21
Play
Full screen (f)
Deck 7: Enzyme Kinetics and Inhibition
1
Which of the following is true about enzymes?
A) enzymes show very little specificity for their substrates
B) enzymes catalyze reactions in only one direction
C) enzyme activities can often be regulated
D) enzymes reaction rates are generally slower than other chemical catalysts
E) enzymes operate under a wide range of temperatures and pH
A) enzymes show very little specificity for their substrates
B) enzymes catalyze reactions in only one direction
C) enzyme activities can often be regulated
D) enzymes reaction rates are generally slower than other chemical catalysts
E) enzymes operate under a wide range of temperatures and pH
enzyme activities can often be regulated
2
How is an enzyme-catalyzed reaction affected by the addition of more enzyme?
A) velocity is not effected
B) velocity will increase only if more substrate is also added
C) velocity will increase
D) velocity will decrease
E) none of the above
A) velocity is not effected
B) velocity will increase only if more substrate is also added
C) velocity will increase
D) velocity will decrease
E) none of the above
velocity will increase
3
For a reaction A + B C, if [B] is much larger than [A] so that [B] essentially remains constant over the course of the reaction, the kinetics will be _____.
A) zero-order
B) hyperbolic
C) first-order
D) sigmoidal
E) pseudo first-order
A) zero-order
B) hyperbolic
C) first-order
D) sigmoidal
E) pseudo first-order
pseudo first-order
4
Which of the following properly expresses the Michaelis-Menten equation?
A) vo = Vmax [S] / (KM + [S])
B) vo = Vmax KM / (KM + [S])
C) kcat = Vmax / [E]T
D) Vmax = vo [S] / (KM + [S])
E) Vmax = vo KM / (KM + [S])
A) vo = Vmax [S] / (KM + [S])
B) vo = Vmax KM / (KM + [S])
C) kcat = Vmax / [E]T
D) Vmax = vo [S] / (KM + [S])
E) Vmax = vo KM / (KM + [S])

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
5
When is KM considered to be the same as the dissociation constant for the ES complex i.e., KM [E] [S] / [ES].
A) ES E + P is fast compared to ES E + S
B) the turnover number is very large
C) kcat/KM is near the diffusion-controlled limit
D) k2 << k-1
E) KM can never be the same as the dissociation constant
A) ES E + P is fast compared to ES E + S
B) the turnover number is very large
C) kcat/KM is near the diffusion-controlled limit
D) k2 << k-1
E) KM can never be the same as the dissociation constant

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
6
What percentage of Vmax is obtained when the substrate is present at ¼ of the KM?
`
A) 5%
B) 20%
C) 25%
D) 80%
E) 100%
`
A) 5%
B) 20%
C) 25%
D) 80%
E) 100%

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following must be true for an enzymatic reaction obeying the Michaelis-Menten equation to reach 3/4 of its maximum velocity?
A) [S] must be ¾KM
B) [S] must be 1.5KM
C) [S] must be 2KM
D) [S] must be 3KM
E) [S] must be 4KM
A) [S] must be ¾KM
B) [S] must be 1.5KM
C) [S] must be 2KM
D) [S] must be 3KM
E) [S] must be 4KM

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following types of enzyme-catalyzed reactions follows non-Michaelis-Menten kinetics?
A) bisubstrate reactions with a random mechanism
B) bisubstrate reactions with a ping pong mechanism
C) bisubstrate reactions with an ordered mechanism
D) allosteric enzyme reactions
E) all of the above
A) bisubstrate reactions with a random mechanism
B) bisubstrate reactions with a ping pong mechanism
C) bisubstrate reactions with an ordered mechanism
D) allosteric enzyme reactions
E) all of the above

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
9
In a bisubstrate reaction, reactant A binds, followed by reactant B which then get converted to products C and D. An experiment showed that B cannot bind without A having bound first. What mechanism is indicated by this data?
A) ordered mechanism
B) random mechanism
C) ping pong mechanism
D) cooperative mechanism
E) none of the above
A) ordered mechanism
B) random mechanism
C) ping pong mechanism
D) cooperative mechanism
E) none of the above

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
10
In a bisubstrate reaction, reactant A binds and is then converted to product C. Next, reactant B binds and is then converted to product D. An experiment showed that B cannot bind without C being released first. What mechanism is indicated by this data?
A) ordered mechanism
B) random mechanism
C) ping pong mechanism
D) cooperative mechanism
E) none of the above
A) ordered mechanism
B) random mechanism
C) ping pong mechanism
D) cooperative mechanism
E) none of the above

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
11
How are the kinetics of an enzyme-catalyzed reaction affected by a competitive inhibitor?
A) Vmax decreased, KM increased
B) Vmax decreased, KM decreased
C) Vmax decreased, KM unchanged
D) Vmax unchanged, KM increased
E) Vmax unchanged, KM decreased
A) Vmax decreased, KM increased
B) Vmax decreased, KM decreased
C) Vmax decreased, KM unchanged
D) Vmax unchanged, KM increased
E) Vmax unchanged, KM decreased

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
12
If a Lineweaver-Burk plot was made for an enzyme-catalyzed reaction, both with and without a competitive inhibitor present, what difference would be seen?
A) the y-intercept would be lower for the inhibited reaction
B) the y-intercept would be higher for the inhibited reaction
C) the slope would be less for the inhibited reaction
D) the slope would be greater for the inhibited reaction
E) none of the above
A) the y-intercept would be lower for the inhibited reaction
B) the y-intercept would be higher for the inhibited reaction
C) the slope would be less for the inhibited reaction
D) the slope would be greater for the inhibited reaction
E) none of the above

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
13
What type of inhibition explains why even at very high substrate concentrations, enzyme activity will decrease as time increases?
A) allosteric inhibition
B) product inhibition
C) transition state analogs
D) irreversible inhibition
E) uncompetitive inhibition
A) allosteric inhibition
B) product inhibition
C) transition state analogs
D) irreversible inhibition
E) uncompetitive inhibition

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is true regarding transition state analogs?
A) they are competitive inhibitors
B) they bind to an active site with much higher affinity than most inhibitors
C) they are much more stable than the transition state
D) their affinity for an enzyme is often much greater that the substrate
E) all of the above
A) they are competitive inhibitors
B) they bind to an active site with much higher affinity than most inhibitors
C) they are much more stable than the transition state
D) their affinity for an enzyme is often much greater that the substrate
E) all of the above

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
15
How are the kinetics of an enzyme-catalyzed reaction affected by a purely noncompetitive inhibitor?
A) Vmax decreased, KM increased
B) Vmax decreased, KM decreased
C) Vmax decreased, KM unchanged
D) Vmax unchanged, KM increased
E) Vmax unchanged, KM decreased
A) Vmax decreased, KM increased
B) Vmax decreased, KM decreased
C) Vmax decreased, KM unchanged
D) Vmax unchanged, KM increased
E) Vmax unchanged, KM decreased

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
16
If a Lineweaver-Burk plot was made for an enzyme-catalyzed reaction, both with and without a noncompetitive inhibitor present, what difference would be seen?
A) the y-intercept would be higher with slope unchanged for the inhibited reaction
B) the y-intercept would be lower with larger slope for the inhibited reaction
C) the y-intercept would be lower with smaller slope for the inhibited reaction
D) the y-intercept would be higher with larger slope for the inhibited reaction
E) the y-intercept would be higher with smaller slope for the inhibited reaction
A) the y-intercept would be higher with slope unchanged for the inhibited reaction
B) the y-intercept would be lower with larger slope for the inhibited reaction
C) the y-intercept would be lower with smaller slope for the inhibited reaction
D) the y-intercept would be higher with larger slope for the inhibited reaction
E) the y-intercept would be higher with smaller slope for the inhibited reaction

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
17
How are the kinetics of an enzyme-catalyzed reaction affected by a mixed inhibitor?
A) Vmax decreased, KM increased or decreased
B) Vmax decreased, KM decreased
C) Vmax decreased, KM increased
D) Vmax unchanged, KM increased
E) Vmax unchanged, KM increased or decreased
A) Vmax decreased, KM increased or decreased
B) Vmax decreased, KM decreased
C) Vmax decreased, KM increased
D) Vmax unchanged, KM increased
E) Vmax unchanged, KM increased or decreased

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
18
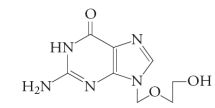
Acyclovir is an analog of ___ that lacks interferes with ___.


Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
19
Find the initial velocity for an enzymatic reaction when Vmax = 6.5 × 10-5 mol•sec-1, [S] = 3.0 × 10-3 M, KM = 4.5 × 10-3 M, and the enzyme concentration at time zero is 1.5 × 10-2 M.
A) 3.9 × 10-5 mol•sec-1
B) 2.6 × 10-5 mol•sec-1
C) 1.4 × 10-2 mol•sec-1
D) 8.7 × 10-3 mol•sec-1
E) Not enough information is given to make this calculation.
A) 3.9 × 10-5 mol•sec-1
B) 2.6 × 10-5 mol•sec-1
C) 1.4 × 10-2 mol•sec-1
D) 8.7 × 10-3 mol•sec-1
E) Not enough information is given to make this calculation.

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
20
The Michaelis constant KM is defined as _____.
I. (k-1 + k2)/k1
II. one-half Vmax
III. [S] = [ES]
IV. [ES]/2
A) I
B) I, II
C) II
D) I, IV
E) II, IV
I. (k-1 + k2)/k1
II. one-half Vmax
III. [S] = [ES]
IV. [ES]/2
A) I
B) I, II
C) II
D) I, IV
E) II, IV

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck
21
Find kcat for a reaction in which Vmax is 4 × 10-4 mol•min-1 and the reaction mixture contains one microgram of enzyme (the molecular weight of the enzyme is 200,000
A) 2 × 10-11 min-1
B) 8 × 107 min-1
C) 8 × 109 min-1
D) 2 × 10-14 min-1
E) 4 × 108 min-1
A) 2 × 10-11 min-1
B) 8 × 107 min-1
C) 8 × 109 min-1
D) 2 × 10-14 min-1
E) 4 × 108 min-1

Unlock Deck
Unlock for access to all 21 flashcards in this deck.
Unlock Deck
k this deck


