Deck 1: The Chemical Basis of Life
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/16
Play
Full screen (f)
Deck 1: The Chemical Basis of Life
1
Of the following amino acids, which contains an alcohol?
A)
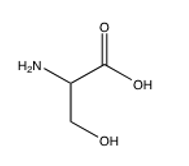
B)
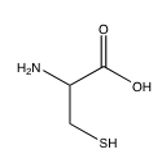
C)
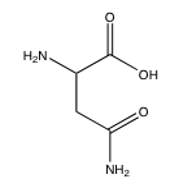
D)
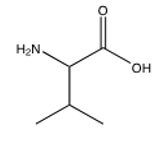
E) all of the above
A)

B)

C)

D)

E) all of the above

2
Which of the major types of biomolecules is never found in a polymeric form?
A) amino acids
B) carbohydrates
C) nucleotides
D) lipids
E) none of the above
A) amino acids
B) carbohydrates
C) nucleotides
D) lipids
E) none of the above
lipids
3
Which of the following biopolymers is correctly paired with the bond that forms between the monomers?
A) protein: ester bond
B) polysaccharide: glycosidic bond
C) DNA: phosphate bond
D) RNA: phosphate bond
E) all of the above
A) protein: ester bond
B) polysaccharide: glycosidic bond
C) DNA: phosphate bond
D) RNA: phosphate bond
E) all of the above
polysaccharide: glycosidic bond
4
Which of the biopolymers is correctly paired with its major function?
A) protein: information encoding
B) nucleic acids: energy storage
C) lipids: information encoding
D) polysaccharide: energy storage
E) none of the above
A) protein: information encoding
B) nucleic acids: energy storage
C) lipids: information encoding
D) polysaccharide: energy storage
E) none of the above

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
5
Entropy is used to measure _____.
A) free energy
B) heat content
C) temperature
D) randomness
E) all of the above
A) free energy
B) heat content
C) temperature
D) randomness
E) all of the above

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
6
A spontaneous process always has _____.
A) G < 0
B) G > 0
C) H < 0
D) H > 0
E) none of the above
A) G < 0
B) G > 0
C) H < 0
D) H > 0
E) none of the above

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
7
An exergonic process _____.
A) occurs without the addition of free energy
B) has a G < 0
C) is spontaneous
D) will have more products than reactants at equilibrium
E) all of the above
A) occurs without the addition of free energy
B) has a G < 0
C) is spontaneous
D) will have more products than reactants at equilibrium
E) all of the above

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
8
If the following two reactions were coupled, what would be the G for the overall exergonic reaction? 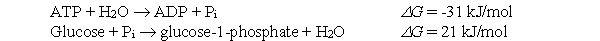
A) -52 kJ/mol
B) -10 kJ/mol
C) 10 kJ/mol
D) 52 kJ/mol
E) none of the above
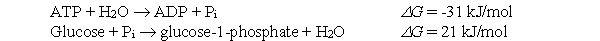
A) -52 kJ/mol
B) -10 kJ/mol
C) 10 kJ/mol
D) 52 kJ/mol
E) none of the above

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
9
A gaseous mixture of hydrogen, water, ammonia and methane can produce which of the biomolecules when exposed to an electrical discharge (such as lightning)?
A) carbohydrates
B) nucleotides
C) lipids
D) amino acids
E) none of the above
A) carbohydrates
B) nucleotides
C) lipids
D) amino acids
E) none of the above

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following explains how nucleotides might have polymerized into nucleic acids in the prebiotic world?
A) a mixture of hydrogen cyanide, formaldehyde and phosphate can form nucleotides in the presence of an electrical discharge
B) nucleotides formed short polymers in the high temperatures of hydrothermal vents
C) nucleotides used the surface of clay as a catalyst to form polymers
D) catalysts such as iron sulfide allow for the formation of new C-C bonds
E) all of the above
A) a mixture of hydrogen cyanide, formaldehyde and phosphate can form nucleotides in the presence of an electrical discharge
B) nucleotides formed short polymers in the high temperatures of hydrothermal vents
C) nucleotides used the surface of clay as a catalyst to form polymers
D) catalysts such as iron sulfide allow for the formation of new C-C bonds
E) all of the above

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
11
Photosynthetic organisms use energy from the sun to reduce _____ to _____.
A) formaldehyde; ethanol
B) CO2; ethanol
C) CO2; carbohydrates
D) CO2; oxygen
E) none of the above
A) formaldehyde; ethanol
B) CO2; ethanol
C) CO2; carbohydrates
D) CO2; oxygen
E) none of the above

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
12
The similarity of one organism to another (for example a bacteria versus a human) is most easily done by comparing which biopolymer?
A) nucleic acids
B) polysaccharides
C) proteins
D) lipids
E) all of the above
A) nucleic acids
B) polysaccharides
C) proteins
D) lipids
E) all of the above

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
13
The biochemical principle that organisms acquire, transform, store, and use energy requires that cells be able to ______.
A) produce their own energy
B) convert light into other forms of energy
C) extract heat from their environment
D) extract energy from their environment
E) use only one form of energy from their environment
A) produce their own energy
B) convert light into other forms of energy
C) extract heat from their environment
D) extract energy from their environment
E) use only one form of energy from their environment

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
14
What are the four most common elements in biological systems? ___; ___; ___; ___

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
15
Name the four major types of biomolecules. ___; ___; ___; ___

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck
16
Based on the information given ( H = 5 kJ/mol and S = 20. J/K•mol that is carried out at 15°C. Please describe the following reaction.
A) spontaneous and endergonic
B) spontaneous and exergonic
C) nonspontaneous and endergonic
D) nonspontaneous and exergonic
E) none of the above
A) spontaneous and endergonic
B) spontaneous and exergonic
C) nonspontaneous and endergonic
D) nonspontaneous and exergonic
E) none of the above

Unlock Deck
Unlock for access to all 16 flashcards in this deck.
Unlock Deck
k this deck


