Deck 9: Acids, Bases, and Buffers in the Body
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/80
Play
Full screen (f)
Deck 9: Acids, Bases, and Buffers in the Body
1
A Brønsted-Lowry acid is defined as a substance which:
A)increases [H+] when placed in water
B)decreases [H+] when placed in water
C)acts as a proton acceptor in any system
D)acts as a proton donor in any system
A)increases [H+] when placed in water
B)decreases [H+] when placed in water
C)acts as a proton acceptor in any system
D)acts as a proton donor in any system
acts as a proton donor in any system
2
A Brønsted-Lowry base is defined as a substance which:
A)acts as proton acceptor in any system
B)acts as proton donor in any system
C)decreases [H+] when placed in water
D)increases [H+] when placed in water
A)acts as proton acceptor in any system
B)acts as proton donor in any system
C)decreases [H+] when placed in water
D)increases [H+] when placed in water
acts as proton acceptor in any system
3
An Arrhenius acid is defined as a substance which:
A)acts as proton acceptor in any system
B)acts as proton donor in any system
C)decreases [H+] when placed in water
D)increases [H+] when placed in water
A)acts as proton acceptor in any system
B)acts as proton donor in any system
C)decreases [H+] when placed in water
D)increases [H+] when placed in water
increases [H+] when placed in water
4
Which of the following properties is not characteristic of an acid?
A)Has a sour taste
B)Produces H+ in water
C)Has a slippery feel
D)Is neutralized by a base
A)Has a sour taste
B)Produces H+ in water
C)Has a slippery feel
D)Is neutralized by a base

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following properties is not characteristic of a base?
A)Has a bitter taste
B)Produces H+ in water
C)Has a slippery feel
D)Is neutralized by an acid
A)Has a bitter taste
B)Produces H+ in water
C)Has a slippery feel
D)Is neutralized by an acid

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
6
In the following equation which is the proton donor and which is the proton acceptor?
CO32- (aq)+ H2O (l)? HCO3- (aq)+ OH- (aq)
A)Donor: CO32-; acceptor: H2O
B)Donor: H2O; acceptor: CO32-
C)Donor: HCO3-; acceptor: OH-
D)Donor: OH-; acceptor: HCO3-
CO32- (aq)+ H2O (l)? HCO3- (aq)+ OH- (aq)
A)Donor: CO32-; acceptor: H2O
B)Donor: H2O; acceptor: CO32-
C)Donor: HCO3-; acceptor: OH-
D)Donor: OH-; acceptor: HCO3-

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following cannot act as a Brønsted-Lowry acid?
A)HSO4-
B)H2O
C)CO32-
D)HS-
A)HSO4-
B)H2O
C)CO32-
D)HS-

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
8
In the following equation identify the Brønsted-Lowry acid and base, respectively:
NH3 + HCN ? NH4+ + CN-
A)NH3 and HCN
B)HCN and NH3
C)NH4+ and CN-
D)NH3 and NH4+
NH3 + HCN ? NH4+ + CN-
A)NH3 and HCN
B)HCN and NH3
C)NH4+ and CN-
D)NH3 and NH4+

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is not a strong acid?
A)HCl (aq)
B)HNO3
C)HC2H3O2
D)HClO4
A)HCl (aq)
B)HNO3
C)HC2H3O2
D)HClO4

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is not a strong base?
A)NaOH
B)Al(OH)3
C)KOH
D)Ca(OH)2
A)NaOH
B)Al(OH)3
C)KOH
D)Ca(OH)2

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is a strong base?
A)NH3
B)CH3OH
C)Ba(OH)2
D)CH3COOH
A)NH3
B)CH3OH
C)Ba(OH)2
D)CH3COOH

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is a weak acid?
A)H3PO4
B)HNO3
C)NH3
D)OH-
A)H3PO4
B)HNO3
C)NH3
D)OH-

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is a correct balanced equation for the neutralization reaction that occurs between Al(OH)3 and HCl (aq)?
A)Al(OH)3 + 3 HCl (aq)? AlCl3 + 3 H2O
B)Al(OH)3 + HCl (aq)? AlCl3 + H2O
C)Al(OH)3 + 3 HCl (aq)? AlCl3 + H+ + OH-
D)Al3+ + OH- + H+ + Cl- ? AlCl3 + H2O
A)Al(OH)3 + 3 HCl (aq)? AlCl3 + 3 H2O
B)Al(OH)3 + HCl (aq)? AlCl3 + H2O
C)Al(OH)3 + 3 HCl (aq)? AlCl3 + H+ + OH-
D)Al3+ + OH- + H+ + Cl- ? AlCl3 + H2O

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
14
The name of the acid HClO is:
A)hypochlorous acid
B)chlorous acid
C)chloric acid
D)perchloric acid
A)hypochlorous acid
B)chlorous acid
C)chloric acid
D)perchloric acid

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following compounds will form CO2 and H2O when it reacts with an acid?
A)Mg(OH)2
B)CaCO3
C)NH3
D)NaC2H3O2
A)Mg(OH)2
B)CaCO3
C)NH3
D)NaC2H3O2

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
16
Given the following reaction, the equilibrium expression will be:
4 CuO (s)+ CH4 (g)? CO2 (g)+ 4 Cu (s)+ 2 H2O (g)
A)[CuO]/[Cu]
B)[CuO]4/[Cu]4
C)[Cu]4/[CuO]4
D)[CO2][H2O]2/[CH4]
4 CuO (s)+ CH4 (g)? CO2 (g)+ 4 Cu (s)+ 2 H2O (g)
A)[CuO]/[Cu]
B)[CuO]4/[Cu]4
C)[Cu]4/[CuO]4
D)[CO2][H2O]2/[CH4]

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
17
The following reaction is exothermic. Which of the following will drive the reaction to the right (towards products)?
CH4 (g)+ 2 O2 (g)? CO2 (g)+ 2 H2O (g)
A)A decrease in temperature
B)An increase in temperature
C)The removal of CH4
D)The addition of CO2
CH4 (g)+ 2 O2 (g)? CO2 (g)+ 2 H2O (g)
A)A decrease in temperature
B)An increase in temperature
C)The removal of CH4
D)The addition of CO2

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
18
Consider the following system at equilibrium: SO2Cl2(g)? SO2(g)+ Cl2(g)
Identify which combination of the following changes will shift the equilibrium to the product side.
I. Addition of SO2Cl2
II. Addition of SO2
III. Removal of SO2Cl2
IV. Removal of Cl2
A)I + II
B)III + IV
C)I + IV
D)II + III
Identify which combination of the following changes will shift the equilibrium to the product side.
I. Addition of SO2Cl2
II. Addition of SO2
III. Removal of SO2Cl2
IV. Removal of Cl2
A)I + II
B)III + IV
C)I + IV
D)II + III

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
19
Consider the following system at equilibrium: N2 (g)+ 3 H2 (g)? 2 NH3 (g)+ 92.94 kJ
Which of the following changes will shift the equilibrium to the right?
1) Increasing the temperature
2) Decreasing the temperature
3) Removing some NH3
4) Adding some NH3
5) Removing some N2
6) Adding some N2
A)2, 3, 6
B)1, 4, 5
C)1, 3, 6
D)2, 4, 6
Which of the following changes will shift the equilibrium to the right?
1) Increasing the temperature
2) Decreasing the temperature
3) Removing some NH3
4) Adding some NH3
5) Removing some N2
6) Adding some N2
A)2, 3, 6
B)1, 4, 5
C)1, 3, 6
D)2, 4, 6

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
20
When a system is at equilibrium:
A)the reaction rate of the forward reaction is equal to the rate of the reverse
B)the reaction rate of the reverse reaction is small compared to forward
C)the reaction rate of the forward reaction is small compared to the reverse
D)the amount of product and reactant is exactly equal
A)the reaction rate of the forward reaction is equal to the rate of the reverse
B)the reaction rate of the reverse reaction is small compared to forward
C)the reaction rate of the forward reaction is small compared to the reverse
D)the amount of product and reactant is exactly equal

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
21
Given the following reaction,write the equilibrium expression:
C (s)+ 2 H2 (g)? CH4 (g)
A)[CH4]/[C] [H2]2
B)[C][H2]
C)[CH4]/[H2]2
D)[H2]2[C]/[CH4]
C (s)+ 2 H2 (g)? CH4 (g)
A)[CH4]/[C] [H2]2
B)[C][H2]
C)[CH4]/[H2]2
D)[H2]2[C]/[CH4]

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
22
What is the conjugate base of OH-?
A)H2O
B)O-
C)O2
D)O-2
A)H2O
B)O-
C)O2
D)O-2

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
23
What is the conjugate acid of HS-?
A)S2-
B)H2S
C)HS-
D)S-
A)S2-
B)H2S
C)HS-
D)S-

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following does not represent a conjugate acid-base pair?
A)H3O+/H2O
B)HCN/CN-
C)HCl/Cl-
D)HC2H3O2/OH-
A)H3O+/H2O
B)HCN/CN-
C)HCl/Cl-
D)HC2H3O2/OH-

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following does not represent a conjugate acid-base pair?
A)HCO3-/CO32-
B)H3PO4/HPO42-
C)OH-/O2-
D)NH4+/NH3
A)HCO3-/CO32-
B)H3PO4/HPO42-
C)OH-/O2-
D)NH4+/NH3

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
26
Which is the correct combination of Brønsted-Lowry bases in the following equilibrium?
H2PO4- + H2O ? H3PO4 + OH-
A)H2PO4-+ OH-
B)H2PO4-+ H3PO4
C)H2O + H3PO4
D)H2O + OH-
H2PO4- + H2O ? H3PO4 + OH-
A)H2PO4-+ OH-
B)H2PO4-+ H3PO4
C)H2O + H3PO4
D)H2O + OH-

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
27
Calculate the pH of 0.00756 M HNO3.
A)11.879
B)7)091
C)2)121
D)12.947
A)11.879
B)7)091
C)2)121
D)12.947

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
28
In an acidic solution, pH is ________ and [H3O+] is ________.
A)= 7, 1 × 10-7 M
B)> 7, < 1 × 10-7 M
C)< 7, > 1 × 10-7 M
D)< 7, < 1 × 10-7 M
A)= 7, 1 × 10-7 M
B)> 7, < 1 × 10-7 M
C)< 7, > 1 × 10-7 M
D)< 7, < 1 × 10-7 M

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
29
In a basic solution, pH is ________ and [H3O+] is ________.
A)= 7, 1 × 10-7 M
B)> 7, < 1 × 10-7 M
C)< 7, > 1 × 10-7 M
D)< 7, < 1 × 10-7 M
A)= 7, 1 × 10-7 M
B)> 7, < 1 × 10-7 M
C)< 7, > 1 × 10-7 M
D)< 7, < 1 × 10-7 M

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
30
What is the pH of a solution that has a [H3O+] = 1.2 × 10-3?
A)1)20
B)2)92
C)11.08
D)12.80
A)1)20
B)2)92
C)11.08
D)12.80

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
31
Calculate the [H3O+] and the pH of a 0.021 M HNO3 solution.
A)4)8 × 10-13 M and 12.32
B)4)8 × 10-13 M and -12.32
C)0)021 M and 1.68
D)0)021 M and -1.68
A)4)8 × 10-13 M and 12.32
B)4)8 × 10-13 M and -12.32
C)0)021 M and 1.68
D)0)021 M and -1.68

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
32
What is the [H3O+] concentration of a solution that has a pH = 2.34?
A)2)3 × 10-3 M
B)4)6 × 10-3 M
C)2)2 × 10-12 M
D)1)2 × 101 M
A)2)3 × 10-3 M
B)4)6 × 10-3 M
C)2)2 × 10-12 M
D)1)2 × 101 M

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
33
What is the [H3O+] concentration of a solution that has a pH = 11.61?
A)1)2 × 101 M
B)1)0 × 10-14 M
C)2)5 × 10-12 M
D)4)1 × 1011 M
A)1)2 × 101 M
B)1)0 × 10-14 M
C)2)5 × 10-12 M
D)4)1 × 1011 M

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following pH's represents a neutral solution?
A)5)40
B)7)00
C)8)65
D)1)25
A)5)40
B)7)00
C)8)65
D)1)25

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is the strongest weak acid?
A)H2PO4- pKa = 7.18
B)NH4+ pKa = 9.20
C)HC2H3O2 pKa = 4.76
D)HCO3- pKa = 10.32
A)H2PO4- pKa = 7.18
B)NH4+ pKa = 9.20
C)HC2H3O2 pKa = 4.76
D)HCO3- pKa = 10.32

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is the weakest acid?
A)H2PO4- pKa = 7.18
B)NH4+ pKa = 9.20
C)HC2H3O2 pKa = 4.76
D)HCO3- pKa = 10.32
A)H2PO4- pKa = 7.18
B)NH4+ pKa = 9.20
C)HC2H3O2 pKa = 4.76
D)HCO3- pKa = 10.32

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
37
Consider the weak acid HCO3- whose pKa = 10.32, which form will predominate when the pH of the solution is 8.5?
A)HCO3-
B)CO32-
C)H2CO3
D)All of these
A)HCO3-
B)CO32-
C)H2CO3
D)All of these

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
38
Consider the weak acid HCO3- whose pKa = 10.32, which form will predominate when the pH of the solution is 12.00?
A)HCO3-
B)CO32-
C)H2CO3
D)All of these
A)HCO3-
B)CO32-
C)H2CO3
D)All of these

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following represents the zwitterions form of the amino acid valine?
A)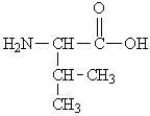
B)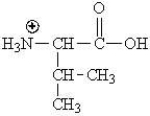
C)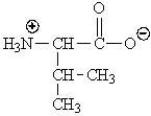
D)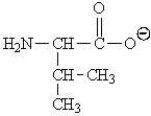
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
40
The isoelectric point of an amino acid is defined as:
A)the pH at which the amino acid exits in the zwitterion form
B)the pH at which it exists in the basic form
C)the pH at which it exists in the acidic form
D)the pH equals the pKa
A)the pH at which the amino acid exits in the zwitterion form
B)the pH at which it exists in the basic form
C)the pH at which it exists in the acidic form
D)the pH equals the pKa

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
41
If the pI for a particular amino acid is 7.5, at which pH will the net charge on the molecule be zero?
A)2)5
B)5)0
C)7)5
D)10.0
A)2)5
B)5)0
C)7)5
D)10.0

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following substances, when added to a solution of nitrous acid (HNO2), could be used to prepare a buffer solution?
A)HCl
B)NaCl
C)HC2H3O2
D)NaNO2
A)HCl
B)NaCl
C)HC2H3O2
D)NaNO2

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following buffers is the one that is mainly contained in our blood?
A)HC2H3O2/C2H3O2-
B)NH4+/NH3
C)H2CO3/HCO3-
D)HPO42-/PO43-
A)HC2H3O2/C2H3O2-
B)NH4+/NH3
C)H2CO3/HCO3-
D)HPO42-/PO43-

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
44
When a person hyperventilates the condition is known as:
A)respiratory acidosis
B)respiratory alkalosis
C)metabolic acidosis
D)metabolic alkalosis
A)respiratory acidosis
B)respiratory alkalosis
C)metabolic acidosis
D)metabolic alkalosis

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
45
When a person does excess vomiting the condition the blood develops is called:
A)respiratory acidosis
B)respiratory alkalosis
C)metabolic acidosis
D)metabolic alkalosis
A)respiratory acidosis
B)respiratory alkalosis
C)metabolic acidosis
D)metabolic alkalosis

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
46
When a diabetic does not have enough glucose in their blood it develops a condition called:
A)respiratory acidosis
B)respiratory alkalosis
C)metabolic acidosis
D)metabolic alkalosis
A)respiratory acidosis
B)respiratory alkalosis
C)metabolic acidosis
D)metabolic alkalosis

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
47
An Arrhenius acid produces ________ ions in aqueous solution.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
48
An Arrhenius base produces ________ ions in aqueous solution.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
49
A Brønsted and Lowry acid ________ protons.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
50
A Brønsted and Lowry base ________ protons.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
51
Identify the acid (A), base (B), conjugate acid (CA)and conjugate base (CB)in each of the following reactions:
A)HSO4- (aq)+ ClO-(aq)⇔ HClO (aq)+ SO42- (aq)
B)H2C2O4 (aq)+ NH3 (aq)⇔ HC2O4- (aq)+ NH4+
C)CN- (aq)+ HC2H3O2 (aq)⇔ HCN (aq)+ C2H3O2-
D)H2S (aq)+ NH3 (aq)⇔ HS- (aq)+ NH4+ (aq)
A)HSO4- (aq)+ ClO-(aq)⇔ HClO (aq)+ SO42- (aq)
B)H2C2O4 (aq)+ NH3 (aq)⇔ HC2O4- (aq)+ NH4+
C)CN- (aq)+ HC2H3O2 (aq)⇔ HCN (aq)+ C2H3O2-
D)H2S (aq)+ NH3 (aq)⇔ HS- (aq)+ NH4+ (aq)

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
52
Identify the weak acids in the following list: HNO3, H2SO3, HF, HCl, H3PO4, H2SO4

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
53
Write the equation that shows how the weak base NH3 produces OH- ions in water.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
54
Insert the coefficients to balance this equation.
________ Na2CO3(s)+ ________ HNO3(aq)→ ________ NaNO3(aq)+ ________ H2O(l)+ ________ CO2(g)
________ Na2CO3(s)+ ________ HNO3(aq)→ ________ NaNO3(aq)+ ________ H2O(l)+ ________ CO2(g)

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
55
The general expression for the equilibrium constant K is given by:



Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
56
A large value of K means that the equilibrium greatly favors the ________, and a small value of K means that the equilibrium greatly favors the ________.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
57
Write the expression for K for the following equilibrium:
4 HCl(aq)+ MnO2(s)⇔ MnCl2(aq)+ Cl2(g)+ 2H2O(l)
4 HCl(aq)+ MnO2(s)⇔ MnCl2(aq)+ Cl2(g)+ 2H2O(l)

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
58
According to Le Chatelier's principle, addition of a reactant shifts the equilibrium to the ________, while addition of a product shifts the equilibrium to the ________.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
59
In the Haber process, in which N2 and H2 are converted into NH3, reduction of the volume at constant temperature causes the amount of NH3 to ________.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
60
The strength of an acid increases as the value of Ka ________.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
61
Use the values of Ka in Table 9.5 in the text to identify the stronger acid in each of these pairs:
A)HF and HNO2
B)NH4+ and HCO3-
C)HCOOH and CH3COOH
A)HF and HNO2
B)NH4+ and HCO3-
C)HCOOH and CH3COOH

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
62
Write the definition of pH.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
63
As the pH value increases, [H3O+] ________.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
64
Indicate whether the following solutions are acidic, basic or neutral. For each concentration calculate the pH of the solution.
A)[H+] = 1.1 × 10-3 M
B)[H+] = 6.0 × 10-11 M
A)[H+] = 1.1 × 10-3 M
B)[H+] = 6.0 × 10-11 M

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
65
Calculate the [H3O+] concentrations in solutions with the following pH values: 2.37, 5.65, 8.92, 12.48

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
66
Write the definition of pKa.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
67
As pKa increases, the strength of the acid ________.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
68
In a solution that has a pH > pKa, the concentration of the acid form is ________ than that of the conjugate base.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
69
The form of an amino acid where the amine group is protonated and the carboxylic group exists an anion is called a ________.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
70
The isoelectric point occurs when the pH of the solution of the amino acid is ________ between the pKa value for the protonated amine and the pKa value for the carboxylic acid.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
71
A Brønsted and Lowry acid accepts protons.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
72
An Arrhenius acid accepts protons.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
73
In the equilibrium HPO42-(aq)+ H2O(l)? H2PO4-(aq)+ OH-(aq), H2O is a Brønsted and Lowry acid.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
74
The equilibrium constant expression for the reaction, 2 HgO(s)? 2 Hg(l)+ O2(g)is K = .
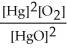
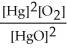

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
75
An equilibrium with a large value of K favors the reactants.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
76
The strength of an acid increases as Ka increases.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
77
When CO2 is bubbled into water at pH 7, the pH decreases.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
78
The strength of an acid increases as pKa increases.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
79
A 1.0 M solution of an acid with a pKa of 5.65 has higher [H3O+] than a 1.0 M solution of an acid with pH of 6.75.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
80
When the pH of a solution of a weak acid is less than the pKa, the concentration of the acid form is greater than that of the conjugate base.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck


