Deck 7: Whats the Attraction State Changes, Solubility, and Lipids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/83
Play
Full screen (f)
Deck 7: Whats the Attraction State Changes, Solubility, and Lipids
1
The weakest attractive force among these is:
A)London force
B)dipole-dipole force
C)hydrogen bonding
D)ion-dipole force
A)London force
B)dipole-dipole force
C)hydrogen bonding
D)ion-dipole force
London force
2
London dispersion forces attractions between molecules depends on what two factors?
A)Molar mass and shape
B)Vapor pressure and size
C)Molar mass and volatility
D)Volatility and shape
A)Molar mass and shape
B)Vapor pressure and size
C)Molar mass and volatility
D)Volatility and shape
Molar mass and shape
3
Which of the following statements about intermolecular forces is true?
A)London dispersions forces are the strongest of the three types.
B)They occur within molecules rather than between the molecules.
C)Hydrogen bonding occurs between any two molecules that contain hydrogen atoms.
D)Dipole-dipole interactions occurs between two polar molecules.
A)London dispersions forces are the strongest of the three types.
B)They occur within molecules rather than between the molecules.
C)Hydrogen bonding occurs between any two molecules that contain hydrogen atoms.
D)Dipole-dipole interactions occurs between two polar molecules.
Dipole-dipole interactions occurs between two polar molecules.
4
When NaCl dissolves in water, the force of attraction that exists between Na+ and H2O is called:
A)dipole-dipole
B)ion-ion
C)hydrogen bonding
D)ion-dipole
A)dipole-dipole
B)ion-ion
C)hydrogen bonding
D)ion-dipole

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
5
________ attractions are the only ones that all molecules have regardless of what they are composed of.
A)Dipole-dipole attractions
B)Hydrogen bonding
C)London dispersion forces
D)Ion-ion interactions
A)Dipole-dipole attractions
B)Hydrogen bonding
C)London dispersion forces
D)Ion-ion interactions

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following compounds cannot exhibit hydrogen bonding?
A)H2O
B)NH3
C)HF
D)CH4
A)H2O
B)NH3
C)HF
D)CH4

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
7
Which one of these molecules can act as a hydrogen bond acceptor but not a donor?
A)CH3-O-CH3
B)C2H5OH
C)CH3NH2
D)CH3CO2H
A)CH3-O-CH3
B)C2H5OH
C)CH3NH2
D)CH3CO2H

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
8
What is the predominant intermolecular force that is between two molecules of CH3CH2OH?
A)London dispersion forces
B)Dipole-dipole
C)Hydrogen bonding
D)Ion-dipole
A)London dispersion forces
B)Dipole-dipole
C)Hydrogen bonding
D)Ion-dipole

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following compounds exhibits primarily dipole-dipole intermolecular forces?
A)CH3-O-CH3
B)CH3CH3
C)CO2
D)F2
A)CH3-O-CH3
B)CH3CH3
C)CO2
D)F2

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
10
How many hydrogen bonds can CH3-O-CH2OH form with water?
A)3
B)4
C)5
D)6
A)3
B)4
C)5
D)6

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
11
For a series of small molecules of comparable molecular weight, which one of the following choices lists the intermolecular forces in the correct increasing order?
A)London forces < dipole-dipole forces < hydrogen bonds
B)Hydrogen bonds < dipole-dipole forces < London forces
C)Dipole-dipole forces < hydrogen bonds < London forces
D)London forces < hydrogen bonds < dipole-dipole forces
A)London forces < dipole-dipole forces < hydrogen bonds
B)Hydrogen bonds < dipole-dipole forces < London forces
C)Dipole-dipole forces < hydrogen bonds < London forces
D)London forces < hydrogen bonds < dipole-dipole forces

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following has only London dispersion forces as the primary attraction between molecules?
A)H2S
B)CH3CH3
C)CH3CH2OH
D)CH3OH
A)H2S
B)CH3CH3
C)CH3CH2OH
D)CH3OH

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following pairs of compounds contain the same intermolecular forces?
A)CH3CH3 and H2O
B)CH3CH2OH and H2O
C)H2S and CH4
D)NH3 and CH4
A)CH3CH3 and H2O
B)CH3CH2OH and H2O
C)H2S and CH4
D)NH3 and CH4

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
14
What is the pressure of nitrogen in atmospheres of a sample that is at 745 mmHg?
A)1)02 atm
B)0)980 atm
C)0)750 atm
D)1)50 atm
A)1)02 atm
B)0)980 atm
C)0)750 atm
D)1)50 atm

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
15
If a scuba diver's lungs have a normal capacity of 4.9 L at sea level (1.0 atm), what would be the volume of her lungs if the pressure at a depth of 50 ft is 975 mmHg?
A)0)26 L
B)477 L
C)6)3 L
D)3)8 L
A)0)26 L
B)477 L
C)6)3 L
D)3)8 L

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
16
A sample of oxygen at room temperature occupies a volume of 500. L at 1.75 atm. What would be the volume of this gas at 2.50 atm at the same temperature?
A)350. L
B)0)00286 L
C)875 L
D)250. L
A)350. L
B)0)00286 L
C)875 L
D)250. L

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
17
What is the new volume of a 3.0 L sample of nitrogen gas that is heated from 75°C to 150°C?
A)1)9 L
B)2)5 L
C)3)6 L
D)5)0 L
A)1)9 L
B)2)5 L
C)3)6 L
D)5)0 L

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
18
What is standard atmospheric pressure (760 mmHg)in inches mercury (in Hg)? (2.54 cm = 1 in)
A)1930 in Hg
B)101 in Hg
C)76.00 in Hg
D)29.92 in Hg
A)1930 in Hg
B)101 in Hg
C)76.00 in Hg
D)29.92 in Hg

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
19
The transition from the gas phase directly to the solid phase is called:
A)condensation
B)freezing
C)sublimation
D)deposition
A)condensation
B)freezing
C)sublimation
D)deposition

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
20
The slow disappearance of a frozen puddle on the sidewalk during winter is due to:
A)sublimation
B)vaporization
C)melting
D)condensation
A)sublimation
B)vaporization
C)melting
D)condensation

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
21
Which of these alkanes has the lowest boiling point?
A)C2H6
B)C4H10
C)C6H14
D)C8H18
A)C2H6
B)C4H10
C)C6H14
D)C8H18

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following compounds will have the lowest boiling point?
A)CH3CH2OH
B)NH3
C)CHCl3
D)CH4
A)CH3CH2OH
B)NH3
C)CHCl3
D)CH4

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following compounds has the highest boiling point?
A)CH4
B)CH3CH3
C)CH3CH2CH2CH3
D)CH3CH2CH3
A)CH4
B)CH3CH3
C)CH3CH2CH2CH3
D)CH3CH2CH3

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following alkanes has the highest boiling point?
A)CH3CH2CH2CH2CH2CH3
B)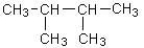
C)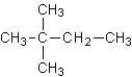
D)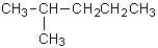
A)CH3CH2CH2CH2CH2CH3
B)

C)

D)
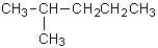

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
25
Let's compare two compounds of similar molar mass in which A is comprised of nonpolar molecules and B composed of polar molecules. Which of the following statements is true?
A)Both compounds have the same boiling point.
B)B will not boil.
C)B will boil at a lower temperature than will A.
D)B will boil at a higher temperature than will A.
A)Both compounds have the same boiling point.
B)B will not boil.
C)B will boil at a lower temperature than will A.
D)B will boil at a higher temperature than will A.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
26
Which compound would have the highest boiling point?
A)CH3-O-CH2CH2CH3
B)CH3CH2-O-CH2CH3
C)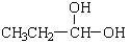
D)CH3CH2CH2CH2-OH
A)CH3-O-CH2CH2CH3
B)CH3CH2-O-CH2CH3
C)
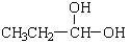
D)CH3CH2CH2CH2-OH

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
27
Which one of the following is most soluble in hexane, C6H14?
A)CH3OH
B)CH3-O-CH3
C)CH3CH2OH
D)CH3CH2CH3
A)CH3OH
B)CH3-O-CH3
C)CH3CH2OH
D)CH3CH2CH3

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following would be most soluble in water?
A)CH3CH2CH3
B)CH3CH2OH
C)CO2
D)CH4
A)CH3CH2CH3
B)CH3CH2OH
C)CO2
D)CH4

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
29
Soaps can be described as:
A)esters of fatty acids
B)salts of fatty acids
C)long chain acids
D)all of these
A)esters of fatty acids
B)salts of fatty acids
C)long chain acids
D)all of these

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
30
The long hydrocarbon tails of soap molecules are:
A)hydrophilic and attracted to water
B)hydrophobic and attracted to water
C)hydrophobic and attracted to oils
D)hydrophilic and attracted to oils
A)hydrophilic and attracted to water
B)hydrophobic and attracted to water
C)hydrophobic and attracted to oils
D)hydrophilic and attracted to oils

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
31
The polar heads of soap molecules are:
A)hydrophilic and attracted to water
B)hydrophobic and attracted to water
C)hydrophobic and attracted to oils
D)hydrophilic and attracted to oils
A)hydrophilic and attracted to water
B)hydrophobic and attracted to water
C)hydrophobic and attracted to oils
D)hydrophilic and attracted to oils

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
32
Fats are generally ________ at room temperature and are obtained from ________.
A)solids; animals
B)liquids; plants
C)solids; plants
D)liquids; animal
A)solids; animals
B)liquids; plants
C)solids; plants
D)liquids; animal

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
33
What chemical process is used to convert oils into fats and semi-solids?
A)Hydration
B)Hydrogenation
C)Saponification
D)Esterification
A)Hydration
B)Hydrogenation
C)Saponification
D)Esterification

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
34
In naturally occurring unsaturated fatty acids the double bonds are:
A)all cis
B)all trans
C)both cis and trans
D)neither cis nor trans
A)all cis
B)all trans
C)both cis and trans
D)neither cis nor trans

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is most likely to be found in the central region of a lipid membrane?
A)Cholesterol
B)Glycine
C)Glucose
D)Methyl phosphate
A)Cholesterol
B)Glycine
C)Glucose
D)Methyl phosphate

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
36
In a lipid bilayer:
A)the hydrophilic heads of the molecules point towards each other
B)all the molecules are triglycerides
C)the hydrophobic heads point to the hydrophilic tails
D)the hydrophobic tails of the molecules point toward each other
A)the hydrophilic heads of the molecules point towards each other
B)all the molecules are triglycerides
C)the hydrophobic heads point to the hydrophilic tails
D)the hydrophobic tails of the molecules point toward each other

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following types of lipids is predominantly found in cell membranes?
A)Waxes
B)Steroids
C)Phospholipids
D)Triglycerides
A)Waxes
B)Steroids
C)Phospholipids
D)Triglycerides

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following statements is not correct about cholesterol?
A)It is an essential component of cell membranes.
B)It is the precursor of steroid hormones.
C)It is soluble in non-polar solvents.
D)It is soluble in polar solvents.
A)It is an essential component of cell membranes.
B)It is the precursor of steroid hormones.
C)It is soluble in non-polar solvents.
D)It is soluble in polar solvents.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
39
Opposite charges attract.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
40
London forces are the strongest type of intermolecular attractive force.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
41
Induced dipole and dispersion forces describe the same force as London forces.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
42
CH3OCH3 can make two donor hydrogen bonds with water.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
43
A salt solution involves predominantly ionic attractions.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
44
A salt bridge involves predominantly ion-dipole attractions.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
45
Hydrogen bonding between bases is responsible for the double helix structure of DNA.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
46
Boyle's law states that, for a fixed quantity of gas at constant temperature, pressure is directly proportional to the volume.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
47
Charles' law states that the volume of a fixed amount of gas at constant pressure is directly proportional to the absolute temperature.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
48
In sublimation a solid is converted into a gas.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
49
In deposition a gas is converted into a liquid.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
50
Stronger attractive forces between molecules in a solid predict lower melting points.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
51
Straight chain alkanes have lower boiling points than branched alkanes of the same size.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
52
Triglycerides don't dissolve in water because they are polar.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
53
Sugar dissolves in water because of the large London forces present in this large molecule.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
54
Amphipathic molecules contain both polar and nonpolar regions.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
55
Which intermolecular force exists between all molecules regardless of polarity?

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
56
Five types of attractive forces between molecules include:

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
57
"Induced dipole" is an equivalent way of saying ________.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
58
Dipole-dipole attractions exist between molecules that have a ________ dipole.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
59
In order to exhibit hydrogen bonding, the H atom must be bonded to one of the three elements:

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
60
How many hydrogen bonds can CH3CH2NHCH2OH make with water, both donor and acceptor?

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
61
The attractions in salt bridges are ________ in nature.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
62
Pressure is defined as ________ per unit ________.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
63
The variables kept constant in Boyle's law are, ________ and ________.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
64
The variables kept constant in Charles' law are, ________ and ________.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
65
When applying Charles' law, the temperature must be expressed in ________.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
66
In Gay-Lussac's law the variables are: ________ and ________.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
67
Convert all the following pressures to atmospheres (atm):
A)10.0 torr
B)75.0 cmHg
C)275 mmHg
A)10.0 torr
B)75.0 cmHg
C)275 mmHg

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
68
A balloon filled with helium gas at 1.00 atm occupies 16.5 L. What volume would the balloon occupy in the upper atmosphere, at a pressure of 0.175 atm?

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
69
In deposition, the ________ state is turned directly into the ________ state.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
70
At the boiling point, the vapor pressure is equal to the ________ pressure.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
71
Stronger attractive forces predict ________ boiling point and ________ vapor pressure.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
72
The boiling points of alkanes increase systematically with their molar mass because of the increase in ________.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
73
Hexane and 2,3-dimethylbutane have the same formula and molar mass but hexane has a boiling point about 11° higher. Explain why this is so.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
74
For the following pairs of molecules, predict which one has the highest boiling point in each case.
A)CH3-O-CH3 and C2H5OH
B)HCl and HI
C)H2O and H2S
D)(CH3)3N and (CH3)2NH
A)CH3-O-CH3 and C2H5OH
B)HCl and HI
C)H2O and H2S
D)(CH3)3N and (CH3)2NH

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
75
Rank the following compounds in order of increasing boiling points:
O2, CH3CH2CH2CH2-OH, CH3CH2CH2CH3, CH3CH2-O-CH3, H2S.
Explain your order.
O2, CH3CH2CH2CH2-OH, CH3CH2CH2CH3, CH3CH2-O-CH3, H2S.
Explain your order.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
76
In predicting solubility, ________ dissolves ________.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
77
A molecule that contains both polar and nonpolar regions is described as ________.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
78
Explain how soap molecules make micelles to remove dirt and grime from objects.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
79
What is the difference between the terms hydrophobic and hydrophilic?

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck
80
Draw a triglyceride with all saturated carbon chains that have 10 carbons in length.

Unlock Deck
Unlock for access to all 83 flashcards in this deck.
Unlock Deck
k this deck


