Deck 21: More About Matter Waves, Photons and Matter Waves
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/18
Play
Full screen (f)
Deck 21: More About Matter Waves, Photons and Matter Waves
1
The principle of complementarity is due to:
A) Einstein
B) Maxwell
C) Newton
D) Bohr
E) Schrodinger
A) Einstein
B) Maxwell
C) Newton
D) Bohr
E) Schrodinger
Bohr
2
The binding energy of an electron in the ground state in a hydrogen atom is about:
A) 13.6 eV
B) 3.4 eV
C) 10.2 eV
D) 1.0 eV
E) 27.2 eV
A) 13.6 eV
B) 3.4 eV
C) 10.2 eV
D) 1.0 eV
E) 27.2 eV
13.6 eV
3
When a hydrogen atom makes the transition from the second excited state to the ground state (at -13.6 eV) the energy of the photon emitted is:
A) 0
B) 1.5 eV
C) 9.1 eV
D) 12.1 eV
E) 13.6 eV
A) 0
B) 1.5 eV
C) 9.1 eV
D) 12.1 eV
E) 13.6 eV
12.1 eV
4
Which of the following sets of quantum numbers is possible for an electron in a hydogen atom?
A) n = 4,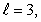
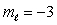
B) n = 4,
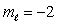
C) n = 5,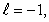
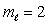
D) n = 3,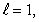
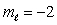
E) n = 2,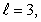
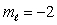
A) n = 4,
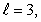
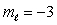
B) n = 4,

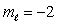
C) n = 5,
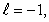
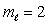
D) n = 3,
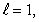
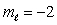
E) n = 2,
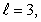
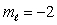

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
5
Consider the following:
I. the probability density for an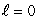 state
state
II. the probability density for a state with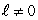
III. the average of the probability densities for all states in an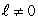 subshell Of these which are spherically symmetric?
subshell Of these which are spherically symmetric?
A) only I
B) only II
C) only I and II
D) only I and III
E) I, II, and III
I. the probability density for an
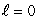 state
stateII. the probability density for a state with
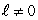
III. the average of the probability densities for all states in an
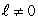 subshell Of these which are spherically symmetric?
subshell Of these which are spherically symmetric?A) only I
B) only II
C) only I and II
D) only I and III
E) I, II, and III

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
6
The radial probability density for the electron in the ground state of a hydrogen atom has a peak at about:
A) 0.5 pm
B) 5 pm
C) 50 pm
D) 500 pm
E) 5000 pm
A) 0.5 pm
B) 5 pm
C) 50 pm
D) 500 pm
E) 5000 pm

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
7
The units of the Planck constant h are those of:
A) energy
B) power
C) momentum
D) angular momentum
E) frequency
A) energy
B) power
C) momentum
D) angular momentum
E) frequency

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
8
The frequency of light beam A is twice that of light beam B. The ratio EA/EB of photon energies is:
A) 1/2
B) 1/4
C) 1
D) 2
E) 4
A) 1/2
B) 1/4
C) 1
D) 2
E) 4

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
9
A photon in light beam A has twice the energy of a photon in light beam B.The ratio pA/pB of their momenta is:
A) 1/2
B) 1/4
C) 1
D) 2
E) 4
A) 1/2
B) 1/4
C) 1
D) 2
E) 4

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
10
Of the following, Compton scattering from electrons is most easily observed for:
A) microwaves
B) infrared light
C) visible light
D) ultraviolet light
E) x rays
A) microwaves
B) infrared light
C) visible light
D) ultraviolet light
E) x rays

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
11
J. J. Thompson's measurement of e/m for electrons provides evidence of the:
A) wave nature of matter
B) particle nature of matter
C) wave nature of radiation
D) particle nature of radiation
E) transverse wave nature of light
A) wave nature of matter
B) particle nature of matter
C) wave nature of radiation
D) particle nature of radiation
E) transverse wave nature of light

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
12
Evidence for the wave nature of matter is:
A) electron diffraction experiments of Davisson and Germer
B) Thompson's measurement of e/m
C) Young's double slit experiment
D) the Compton effect
E) Lenz's law
A) electron diffraction experiments of Davisson and Germer
B) Thompson's measurement of e/m
C) Young's double slit experiment
D) the Compton effect
E) Lenz's law

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
13
Consider the following three particles:
1)a free electron with speed v0
2)a free proton with speed v0
3)a free proton with speed 2v0
Rank them according to the wavlengths of their matter waves, least to greatest.
A) 1, 2, 3
B) 3, 2, 1
C) 2, 3, 1
D) 1, 3, 2
E) 1, then 2 and 3 tied
1)a free electron with speed v0
2)a free proton with speed v0
3)a free proton with speed 2v0
Rank them according to the wavlengths of their matter waves, least to greatest.
A) 1, 2, 3
B) 3, 2, 1
C) 2, 3, 1
D) 1, 3, 2
E) 1, then 2 and 3 tied

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
14
Consider the following three particles:
1)a free electron with kinetic energy K0
2)a free proton with kinetic energy K0
3)a free proton with kinetic energy 2K0
Rank them according to the wavelengths of their waves, least to greatest.
A) 1, 2, 3
B) 3, 2, 1
C) 2, 3, 1
D) 1, 3, 2
E) 1, then 2 and 3 tied
1)a free electron with kinetic energy K0
2)a free proton with kinetic energy K0
3)a free proton with kinetic energy 2K0
Rank them according to the wavelengths of their waves, least to greatest.
A) 1, 2, 3
B) 3, 2, 1
C) 2, 3, 1
D) 1, 3, 2
E) 1, then 2 and 3 tied

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
15
A non-relativistic free electron has kinetic energy K.If its wavelength doubles, its kinetic energy is:
A) 4 K
B) 2 K
C) still K
D) K/2
E) K/4
A) 4 K
B) 2 K
C) still K
D) K/2
E) K/4

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
16
The probability that a particle is in a given small region of space is proportional to:
A) its energy
B) its momentum
C) the frequency of its wave function
D) the wavelength of its wave function
E) the square of the magnitude of its wave function
A) its energy
B) its momentum
C) the frequency of its wave function
D) the wavelength of its wave function
E) the square of the magnitude of its wave function

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
17
Maxwell's equations are to electric and magnetic fields as __________ equation is to the wave function of the particle.
A) Einstein's
B) Fermi's
C) Newton's
D) Schrodinger's
E) Bohr's
A) Einstein's
B) Fermi's
C) Newton's
D) Schrodinger's
E) Bohr's

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck
18
In order to tunnel through a potential barrier a particle must:
A) have energy greater than the barrier height
B) have spin
C) be massive
D) have a wavelength longer than the barrier width
E) none of the above
A) have energy greater than the barrier height
B) have spin
C) be massive
D) have a wavelength longer than the barrier width
E) none of the above

Unlock Deck
Unlock for access to all 18 flashcards in this deck.
Unlock Deck
k this deck


