Deck 4: The Kinetic Theory of Gases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/32
Play
Full screen (f)
Deck 4: The Kinetic Theory of Gases
1
An ideal gas occupies 12 liters at 293 K and 1 atm (76 cm Hg). Its temperature is now raised to 373 K and its pressure increased to 215 cm Hg. The new volume is:
A) 0.2 liters
B) 5.4 liters
C) 13.6 liters
D) 20.8 liters
E) none of these
A) 0.2 liters
B) 5.4 liters
C) 13.6 liters
D) 20.8 liters
E) none of these
5.4 liters
2
An air bubble doubles in volume as it rises from the bottom of a lake (1000 kg/m3). Ignoring any temperature changes, the depth of the lake is:
A) 21 m
B) 0.76 m
C) 4.9 m
D) 10 m
E) 0.99 m
A) 21 m
B) 0.76 m
C) 4.9 m
D) 10 m
E) 0.99 m
10 m
3
An isothermal process for an ideal gas is represented on a p-V diagram by:
A) a horizontal line
B) a vertical line
C) a portion of an ellipse
D) a portion of a parabola
E) a hyperbola
A) a horizontal line
B) a vertical line
C) a portion of an ellipse
D) a portion of a parabola
E) a hyperbola
a hyperbola
4
A real gas is changed slowly from state 1 to state 2. During this process no work is done on or by the gas. This process must be:
A) isothermal
B) adiabatic
C) isovolumic
D) isobaric
E) a closed cycle with point 1 coinciding with point 2
A) isothermal
B) adiabatic
C) isovolumic
D) isobaric
E) a closed cycle with point 1 coinciding with point 2

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
5
In order that a single process be both isothermal and isobaric:
A) one must use an ideal gas
B) such a process is impossible
C) a change of phase is essential
D) one may use any real gas such as N2
E) one must use a solid
A) one must use an ideal gas
B) such a process is impossible
C) a change of phase is essential
D) one may use any real gas such as N2
E) one must use a solid

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
6
The internal energy of an ideal gas depends on:
A) the temperature only
B) the pressure only
C) the volume only
D) the temperature and pressure only
E) temperature, pressure, and volume
A) the temperature only
B) the pressure only
C) the volume only
D) the temperature and pressure only
E) temperature, pressure, and volume

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
7
The mean free path of air molecules at room temperature and atmospheric pressure is about:
A) 10-3 m
B) 10-5 m
C) 10-7 m
D) 10-9 m
E) 10-11 m
A) 10-3 m
B) 10-5 m
C) 10-7 m
D) 10-9 m
E) 10-11 m

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
8
The mean free path of molecules in a gas is proportional to:
A) the molecular diameter
B) the reciprocal of the molecular diameter
C) the molecular concentration
D) the reciprocal of the molecular concentration
E) the average molecular speed
A) the molecular diameter
B) the reciprocal of the molecular diameter
C) the molecular concentration
D) the reciprocal of the molecular concentration
E) the average molecular speed

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
9
A certain ideal gas has a temperature 300 K and a pressure 5.0 x104 Pa.The molecules have a mean free path of 4.0 x10-7m.If the temperature is raised to 350 K and the pressure is reduced to 1.0 x 104 Pa the mean free path is them:
A) 6.9 x 10-8m
B) 9.3 x10-8m
C) 3.3 x10-7m
D) 1.7 x10-6m
E) 2.3 x10-6m
A) 6.9 x 10-8m
B) 9.3 x10-8m
C) 3.3 x10-7m
D) 1.7 x10-6m
E) 2.3 x10-6m

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
10
Evidence that molecules of a gas are in constant motion is:
A) winds exert pressure
B) two gases interdiffuse quickly
C) warm air rises
D) energy as heat is needed to vaporize a liquid
E) gases are easily compressed
A) winds exert pressure
B) two gases interdiffuse quickly
C) warm air rises
D) energy as heat is needed to vaporize a liquid
E) gases are easily compressed

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
11
The mass of an oxygen molecule is 16 times that of a hydrogen molecule. At room temperature, the ratio of the rms speed of an oxygen molecule to that of a hydrogen molecule is:
A) 16
B) 4
C) 1
D) 1/4
E) 1/16
A) 16
B) 4
C) 1
D) 1/4
E) 1/16

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
12
Five molecules have speeds of 2.8, 3.2, 5.8, 7.3, and 7.4 m/s. Their root-mean-square speed is closest to:
A) 5.3 m/s
B) 5.7 m/s
C) 7.3 m/s
D) 28 m/s
E) 32 m/s
A) 5.3 m/s
B) 5.7 m/s
C) 7.3 m/s
D) 28 m/s
E) 32 m/s

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
13
In a system of N gas molecules, the individual speeds are v1, v2, ..., vN. The rms speed of these molecules is:
A)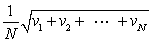
B)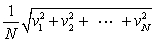
C)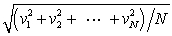
D)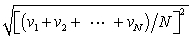
E)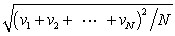
A)
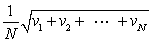
B)
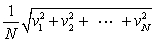
C)
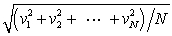
D)

E)


Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
14
The Maxwellian speed distribution provides a direct explanation of:
A) thermal expansion
B) the ideal gas law
C) heat
D) evaporation
E) boiling
A) thermal expansion
B) the ideal gas law
C) heat
D) evaporation
E) boiling

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
15
According to the Maxwellian speed distribution, as the temperature increases the average speed:
A) increases
B) decreases
C) increases at high temperatures and decreases at low
D) decreases at high temperatures and increases at low
E) stays the same
A) increases
B) decreases
C) increases at high temperatures and decreases at low
D) decreases at high temperatures and increases at low
E) stays the same

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
16
As the pressure in an ideal gas is increased isothermally the average molecular speed:
A) increases
B) decreases
C) increases at high temperature, decreases at low
D) decreases at high temperature, increases at low
E) stays the same
A) increases
B) decreases
C) increases at high temperature, decreases at low
D) decreases at high temperature, increases at low
E) stays the same

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
17
As the volume of an ideal gas is increased at constant pressure the average molecular speed:
A) increases
B) decreases
C) increases at high temperature, decreases at low
D) decreases at high temperature, increases at low
E) stays the same
A) increases
B) decreases
C) increases at high temperature, decreases at low
D) decreases at high temperature, increases at low
E) stays the same

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
18
An ideal gas of N diatomic molecules has temperature T. If the number of molecules is doubled without changing the temperature, the internal energy increases by:
A) 0
B) 1/2NkT
C) 3/2NkT
D) 5/2NkT
E) 3NkT
A) 0
B) 1/2NkT
C) 3/2NkT
D) 5/2NkT
E) 3NkT

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
19
The difference between the molar specific heat at constant pressure and the molar specific heat at constant volume for an ideal gas is:
A) the Boltzmann constant k
B) the universal gas constant R
C) the Avogadro number NA
D) kT
E) RT
A) the Boltzmann constant k
B) the universal gas constant R
C) the Avogadro number NA
D) kT
E) RT

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
20
An ideal monatomic gas has a molar specific heat Cv at constant volume of:
A) R
B) 3R/2
C) 5R/2
D) 7R/2
E) 9R/2
A) R
B) 3R/2
C) 5R/2
D) 7R/2
E) 9R/2

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
21
The specific heat Cv at constant volume of a monatomic gas at low pressure is proportional to Tn where the exponent n is:
A) -1
B) 0
C) 1
D) 1/2
E) 2
A) -1
B) 0
C) 1
D) 1/2
E) 2

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
22
The ratio of the specific heat of a gas at constant volume to its specific heat at constant pressure is:
A) 1
B) less than 1
C) more than 1
D) has units of pressure/volume
E) has units of volume/pressure
A) 1
B) less than 1
C) more than 1
D) has units of pressure/volume
E) has units of volume/pressure

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
23
The ratio of the specific heat of an ideal gas at constant volume to its specific heat at constant pressure is:
A) R
B) 1/R
C) dependent on the temperature
D) dependent on the pressure
E) different for monatomic, diatomic, and polyatomic gases
A) R
B) 1/R
C) dependent on the temperature
D) dependent on the pressure
E) different for monatomic, diatomic, and polyatomic gases

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
24
Both the pressure and volume of an ideal gas of diatomic molecules are doubled. The ratio of the new internal energy to the old both measured relative to the internal energy at 0 K is:
A) 1/4
B) 1/2
C) 1
D) 2
E) 4
A) 1/4
B) 1/2
C) 1
D) 2
E) 4

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
25
The pressure of an ideal gas of diatomic molecules is doubled by halving the volume. The ratio of the new internal energy to the old, both measured relative to the internal energy at 0 K, is:
A) 1/4
B) 1/2
C) 1
D) 2
E) 4
A) 1/4
B) 1/2
C) 1
D) 2
E) 4

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
26
The number of degrees of freedom of a rigid diatomic molecule is:
A) 2
B) 3
C) 4
D) 5
E) 6
A) 2
B) 3
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
27
The number of degrees of freedom of a triatomic molecule is:
A) 1
B) 3
C) 6
D) 8
E) 9
A) 1
B) 3
C) 6
D) 8
E) 9

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
28
An ideal diatomic gas has a molar specific heat at constant pressure, Cp, of:
A) R
B) 3R/2
C) 5R/2
D) 7R/2
E) 9R/2
A) R
B) 3R/2
C) 5R/2
D) 7R/2
E) 9R/2

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
29
When work W is done on an ideal gas of N diatomic molecules in thermal isolation the temperature increases by:
A) W/2Nk
B) W/3Nk
C) 2W/3Nk
D) 2W/5Nk
E) W/Nk
A) W/2Nk
B) W/3Nk
C) 2W/3Nk
D) 2W/5Nk
E) W/Nk

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
30
When work W is done on an ideal gas of diatomic molecules in thermal isolation the increase in the total rotational energy of the molecules is:
A) 0
B) W/3
C) 2W/3
D) 2W/5
E) W
A) 0
B) W/3
C) 2W/3
D) 2W/5
E) W

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
31
When work W is done on an ideal gas of diatomic molecules in thermal isolation the increase in the total translational kinetic energy of the molecules is:
A) 0
B) 2W/3
C) 2W/5
D) 3W/5
E) W
A) 0
B) 2W/3
C) 2W/5
D) 3W/5
E) W

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck
32
The heat capacity at constant volume of an ideal gas depends on:
A) the temperature
B) the pressure
C) the volume
D) the number of molecules
E) none of the above
A) the temperature
B) the pressure
C) the volume
D) the number of molecules
E) none of the above

Unlock Deck
Unlock for access to all 32 flashcards in this deck.
Unlock Deck
k this deck


