Deck 7: Making and Breaking of Bonds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/69
Play
Full screen (f)
Deck 7: Making and Breaking of Bonds
1
The following questions often assume that a truncated table of standard-state thermodynamic data is attached to the exam.
Specific Heat and Heat Capacity
-The same amount of heat is added to 20 gram each of iron and water. The temperature increases more in the iron than in the water. Which has the greater specific heat? Explain.
Specific Heat and Heat Capacity
-The same amount of heat is added to 20 gram each of iron and water. The temperature increases more in the iron than in the water. Which has the greater specific heat? Explain.
Water has the greater specific heat. Specific heat is the measure of the amount of heat required to raise the temperature by 1oC of 1 g of a substance. A greater specific heat indicates that more heat will be needed to give a particular increase in the temperature of a substance relative to a substance with a lower specific heat.
2
The following questions often assume that a truncated table of standard-state thermodynamic data is attached to the exam.
Specific Heat and Heat Capacity
-Metal A at 40oC is brought into contact with Metal B at 20oC. Assuming you have the same masses of both metals and that Metal A has a greater specific heat than Metal B, what will the final temperature be?
A) 30oC
B) between 20oC and 30oC
C) between 30oC and 40oC
D) 40oC
E) insufficient information given to decide
Specific Heat and Heat Capacity
-Metal A at 40oC is brought into contact with Metal B at 20oC. Assuming you have the same masses of both metals and that Metal A has a greater specific heat than Metal B, what will the final temperature be?
A) 30oC
B) between 20oC and 30oC
C) between 30oC and 40oC
D) 40oC
E) insufficient information given to decide
between 30oC and 40oC
3
The following questions often assume that a truncated table of standard-state thermodynamic data is attached to the exam.
Specific Heat and Heat Capacity
-When 2.50 g of KClO4 decomposes, 908 J of heat is evolved. If this heat is absorbed by 45.0 g of H2O, what will be the temperature change of the water?
A) 0.207oC decrease
B) 4.82oC increase
C) 6.41oC increase
D) 0.207oC increase
E) none of these
Specific Heat and Heat Capacity
-When 2.50 g of KClO4 decomposes, 908 J of heat is evolved. If this heat is absorbed by 45.0 g of H2O, what will be the temperature change of the water?
A) 0.207oC decrease
B) 4.82oC increase
C) 6.41oC increase
D) 0.207oC increase
E) none of these
4.82oC increase
4
The following questions often assume that a truncated table of standard-state thermodynamic data is attached to the exam.
Specific Heat and Heat Capacity
-How much energy in joules is required to heat 10.0 grams of gold from room temperature (20.0°C) to the temperature of boiling water (100.0°C) if the specific heat of gold is 0.129
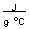 ?
?
A) 1.29 J
B) 10.3 J
C) 103 J
D) 6,200 J
E) none of the above
Specific Heat and Heat Capacity
-How much energy in joules is required to heat 10.0 grams of gold from room temperature (20.0°C) to the temperature of boiling water (100.0°C) if the specific heat of gold is 0.129
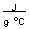 ?
?A) 1.29 J
B) 10.3 J
C) 103 J
D) 6,200 J
E) none of the above

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
5
The following questions often assume that a truncated table of standard-state thermodynamic data is attached to the exam.
Specific Heat and Heat Capacity
-A piece of copper metal weighing 145 grams was heated to 100°C and then dropped into 250 grams of water at 25°C. The copper metal cooled down and the water became warmer until both were at a temperature of 28.8°C. Calculate the amount of heat absorbed by the water. Assuming that the heat lost by the copper was absorbed by the water, what is the molar heat capacity of copper metal?
(CH2O = 75.376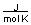 )
)
A) between 0 and 10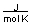
B) between 10 and 20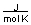
C) between 20 and 30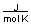
D) between 30 and 40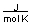
E) more than 40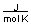
Specific Heat and Heat Capacity
-A piece of copper metal weighing 145 grams was heated to 100°C and then dropped into 250 grams of water at 25°C. The copper metal cooled down and the water became warmer until both were at a temperature of 28.8°C. Calculate the amount of heat absorbed by the water. Assuming that the heat lost by the copper was absorbed by the water, what is the molar heat capacity of copper metal?
(CH2O = 75.376
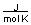 )
)A) between 0 and 10
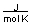
B) between 10 and 20
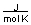
C) between 20 and 30
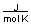
D) between 30 and 40
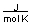
E) more than 40
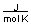

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
6
The following questions often assume that a truncated table of standard-state thermodynamic data is attached to the exam.
Specific Heat and Heat Capacity
-How much heat in joules is produced by mixing 50.0 mL of 1.0 M HBr at 25.6°C with 50.0 mL of 1.0 M KOH at 25.6°C if this reaction produces 100 mL of a solution with a temperature of 32.3°C? (Assume the heat capacity of water is 4.18
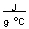 and the density of these solutions is 1.00
and the density of these solutions is 1.00 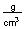 )
)
A) 6.7 J
B) 670 J
C) 1400 J
D) 2800 J
E) 5600 J
Specific Heat and Heat Capacity
-How much heat in joules is produced by mixing 50.0 mL of 1.0 M HBr at 25.6°C with 50.0 mL of 1.0 M KOH at 25.6°C if this reaction produces 100 mL of a solution with a temperature of 32.3°C? (Assume the heat capacity of water is 4.18
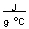 and the density of these solutions is 1.00
and the density of these solutions is 1.00 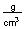 )
)A) 6.7 J
B) 670 J
C) 1400 J
D) 2800 J
E) 5600 J

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
7
The change in enthalpy, H, for the following balanced reaction is 33.2 kJ/molrxn. How much heat will be absorbed by the reaction of 1.00 g of N2 with an excess of oxygen?
N2(g) + 2O2(g) 2 NO2(g)
A) 1.19 kJ
B) 2.37 kJ
C) 33.2 kJ
D) 66.4 kJ
E) none of these
N2(g) + 2O2(g) 2 NO2(g)
A) 1.19 kJ
B) 2.37 kJ
C) 33.2 kJ
D) 66.4 kJ
E) none of these

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following is a correct statement for a chemical reaction that gives off energy? The reaction is:
A) exothermic with a + H
B) exothermic with a - ) H
C) endothermic with a + H
D) endothermic with a - H
E) exothermic with a H=0
A) exothermic with a + H
B) exothermic with a - ) H
C) endothermic with a + H
D) endothermic with a - H
E) exothermic with a H=0

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
9
For the reaction
MnO2(s) + CO(g) MnO(s) + CO2(g) H = -151 kJ/ molrxn.
How many moles of MnO2 are required in order for 418 kJ of heat to be released?
A) 1.31 moles
B) 0.246 moles
C) 0.361 moles
D) 2.77 moles
E) none of these
MnO2(s) + CO(g) MnO(s) + CO2(g) H = -151 kJ/ molrxn.
How many moles of MnO2 are required in order for 418 kJ of heat to be released?
A) 1.31 moles
B) 0.246 moles
C) 0.361 moles
D) 2.77 moles
E) none of these

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
10
How much heat is produced when 0.200 mole of H2(g) reacts with 0.300 mole of Cl2(g) if the enthalpy of reaction for the production of one mole of HCl(g) is -92.3 kJ/molrxn?
A) 18.5 kJ
B) 27.7 kJ
C) 36.9 kJ
D) 55.4 kJ
E) 92.3 kJ
A) 18.5 kJ
B) 27.7 kJ
C) 36.9 kJ
D) 55.4 kJ
E) 92.3 kJ

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
11
How much heat in kilojoules is given off when 4.80 g of carbon are burned to produce carbon dioxide, if 0Hrxn° for the combustion of carbon to form CO2 is -394 kJ/molrxn?
A) 82.1 kJ
B) 158 kJ
C) 394 kJ
D) 985 kJ
E) 1.89 x 103 kJ
A) 82.1 kJ
B) 158 kJ
C) 394 kJ
D) 985 kJ
E) 1.89 x 103 kJ

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
12
Calculate wing reaction if 1.00 gram of magnesium reacts with excess fluorine to give off 46.22 kJ of heat.
Mg(s) + F2(g) MgF2(s)
A) less than 300 kJ/molrxn
B) between 300 and 600 kJ/molrxn
C) between 600 and 900 kJ/molrxn
D) between 900 and 1200 kJ/molrxn
E) more than 1200 kJ/molrxn
Mg(s) + F2(g) MgF2(s)
A) less than 300 kJ/molrxn
B) between 300 and 600 kJ/molrxn
C) between 600 and 900 kJ/molrxn
D) between 900 and 1200 kJ/molrxn
E) more than 1200 kJ/molrxn

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
13
For which of the following reactions is the most heat evolved?
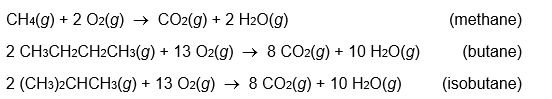
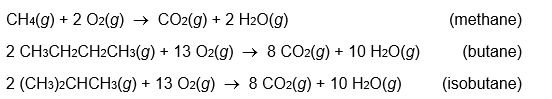

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following reactions is the most likely to give off heat?
A) Al(s) Al(g)
B) Al(g) Al3+(g) + 3 e-
C) Al-(g) Al(g) + e-
D) 2 Al(s) + conc. HNO3(aq) 2 Al3+(aq) + 3 H2(g)
E) all these reactions should give off energy.
A) Al(s) Al(g)
B) Al(g) Al3+(g) + 3 e-
C) Al-(g) Al(g) + e-
D) 2 Al(s) + conc. HNO3(aq) 2 Al3+(aq) + 3 H2(g)
E) all these reactions should give off energy.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following reactions is the most likely to give off heat?
A) Mg(s) Mg(g)
B) Mg(g) Mg2+(g) + 2 e-
C) MgCl2(s) Mg2+(g) + 2 Cl-(g)
D) Mg(g) + 2 Cl(g) MgCl2
E) all these reactions should give off energy
A) Mg(s) Mg(g)
B) Mg(g) Mg2+(g) + 2 e-
C) MgCl2(s) Mg2+(g) + 2 Cl-(g)
D) Mg(g) + 2 Cl(g) MgCl2
E) all these reactions should give off energy

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following reactions is the most likely to be exothermic?
A) H2(g) 2 H(g)
B) 2 F(g) F2(g)
C) C(s) C(g)
D) CCl4(g) C(g) + 4 Cl(g)
E) HCl(g) H(g) + Cl(g)
A) H2(g) 2 H(g)
B) 2 F(g) F2(g)
C) C(s) C(g)
D) CCl4(g) C(g) + 4 Cl(g)
E) HCl(g) H(g) + Cl(g)

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following reactions is the most likely to be exothermic?
A) CaCO3(s) CaO(s) + CO2(g)
B) Ca(g) Ca2+(g) + 2 e-
C) Cl2(g) 2 Cl(g)
D) Cl(g) + e- Cl-(g)
E) CaCl2(g) Ca2+(g) + 2 Cl-(g)
A) CaCO3(s) CaO(s) + CO2(g)
B) Ca(g) Ca2+(g) + 2 e-
C) Cl2(g) 2 Cl(g)
D) Cl(g) + e- Cl-(g)
E) CaCl2(g) Ca2+(g) + 2 Cl-(g)

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following reactions is the most likely to be endothermic?
A) 2 Na(s) + 2 H2O(l) 2 Na+(aq) + 2 OH-(aq) + H2(g)
B) 2 Mg(s) + O2(g) 2 MgO(s)
C) 2 NaCl(s) 2 Na(s) + Cl2(g)
D) Na+(g) + e- Na(g)
E) Cl2(g) + 2e- 2 Cl-(g)
A) 2 Na(s) + 2 H2O(l) 2 Na+(aq) + 2 OH-(aq) + H2(g)
B) 2 Mg(s) + O2(g) 2 MgO(s)
C) 2 NaCl(s) 2 Na(s) + Cl2(g)
D) Na+(g) + e- Na(g)
E) Cl2(g) + 2e- 2 Cl-(g)

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following reactions is the most likely to be endothermic?
A) Mg(s) Mg(g)
B) Na+(g) + Cl-(g) NaCl(s)
C) Na+(g) + e- Na(g)
D) H+(aq) + OH-(aq) H2O(l)
E) 2 H2(g) + O2(g) 2 H2O(l)
A) Mg(s) Mg(g)
B) Na+(g) + Cl-(g) NaCl(s)
C) Na+(g) + e- Na(g)
D) H+(aq) + OH-(aq) H2O(l)
E) 2 H2(g) + O2(g) 2 H2O(l)

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
20
Use your understanding of the bonding in the reactants and products to explain why the following reaction is exothermic.
2 Mg(s) + O2(g) 2 MgO(s)
2 Mg(s) + O2(g) 2 MgO(s)

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
21
At what temperature are standard-state enthalpy of reaction measurements most often made?
A) 0 K
B) 273.15 K
C) 0°C
D) 25°C
E) more than one of the above
A) 0 K
B) 273.15 K
C) 0°C
D) 25°C
E) more than one of the above

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
22
The bond strength between C and a halogen decreases down the halogen group in the periodic table. Based on this trend, predict which of the following gases has the largest (most negative) enthalpy of atom combination?
A) CCl4
B) CBr4
C) CF4
D) CI4
E) They all have the same enthalpy of atom combination
A) CCl4
B) CBr4
C) CF4
D) CI4
E) They all have the same enthalpy of atom combination

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
23
When carbon is burned in air the following reaction takes place and releases heat.
C(s) + O2(g) CO2(g)
Which of the following is responsible for the heat produced?
A) breaking oxygen-oxygen bonds
B) making carbon-oxygen bonds
C) breaking carbon-carbon bonds
D) both (a) and (c)
E) (a), (b) and (c)
C(s) + O2(g) CO2(g)
Which of the following is responsible for the heat produced?
A) breaking oxygen-oxygen bonds
B) making carbon-oxygen bonds
C) breaking carbon-carbon bonds
D) both (a) and (c)
E) (a), (b) and (c)

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is an exothermic process?
A) Li(g) Li+ (g) + e-
B) NH3(g) N (g) + 3 H
C) C(g) + 2 O(g) CO2(g)
D) all of these
E) none of these
A) Li(g) Li+ (g) + e-
B) NH3(g) N (g) + 3 H
C) C(g) + 2 O(g) CO2(g)
D) all of these
E) none of these

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
25
Determine the change in enthalpy for the following reaction:
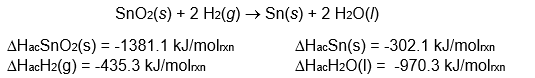
A) -3088 kJ/molrxn
B) +544 kJ/molrxn
C) -544 kJ/molrxn
D) +9.0 kJ/molrxn
E) none of these
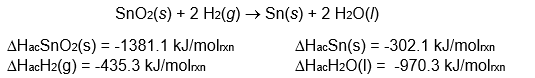
A) -3088 kJ/molrxn
B) +544 kJ/molrxn
C) -544 kJ/molrxn
D) +9.0 kJ/molrxn
E) none of these

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
26
Use enthalpies of atom combination to calculate Hrxn° for the following reaction.
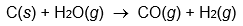
What would happen to the magnitude of Hrxn° if the reaction consumed liquid water instead of gaseous water?

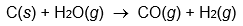
What would happen to the magnitude of Hrxn° if the reaction consumed liquid water instead of gaseous water?


Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
27
Calculate Hrxn° for the following reaction from the data given below.
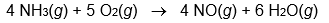
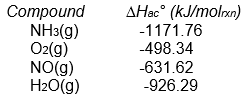
A) -105.5 kJ/molrxn
B) -905.5 kJ/molrxn
C) -1274.2 kJ/molrxn
D) -1996.2 kJ/molrxn
E) none of the above
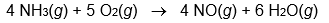
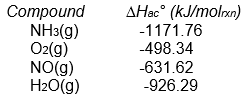
A) -105.5 kJ/molrxn
B) -905.5 kJ/molrxn
C) -1274.2 kJ/molrxn
D) -1996.2 kJ/molrxn
E) none of the above

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
28
Calculate Hrxn° for the reaction:
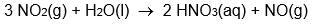
from the following enthalpy of atom combination data.
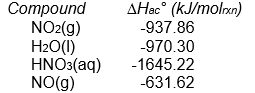
A) less than -1000 kJ/molrxn
B) between -1000 and -750 kJ/molrxn
C) between -750 and -500 kJ/molrxn
D) between -500 and -250 kJ/molrxn
E) between -250 and 0 kJ/molrxn
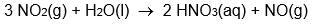
from the following enthalpy of atom combination data.
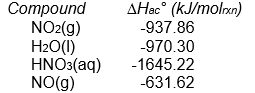
A) less than -1000 kJ/molrxn
B) between -1000 and -750 kJ/molrxn
C) between -750 and -500 kJ/molrxn
D) between -500 and -250 kJ/molrxn
E) between -250 and 0 kJ/molrxn

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
29
Both ethanol (CH3CH2OH) and methanol (CH3OH) have been considered as fuels for automobiles. Which is the better fuel, on a per gram basis, when burned with oxygen?
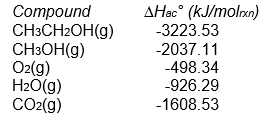
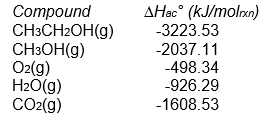

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
30
The following reaction occurs when sucrose (cane sugar) is metabolized by the body.
C12H22O11(s) + 12 O2(g) 12 CO2(g) + 11 H2O(l)
Assume that Hrxn° for this reaction is -5645 kJ/molrxn. What is the value of Hac° for sucrose?
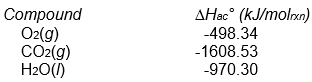
C12H22O11(s) + 12 O2(g) 12 CO2(g) + 11 H2O(l)
Assume that Hrxn° for this reaction is -5645 kJ/molrxn. What is the value of Hac° for sucrose?
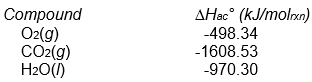

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
31
What is the sign of the enthalpy of reaction for the reaction:
P4O6(s) + 2 O2(g) + 6 H2O(g) 4 H3PO4(s)
Assuming that all compounds are present in their most stable state at 25°C and 1 atm pressure?
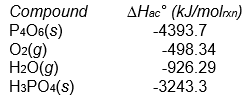
A) positive
B) negative
C) impossible to determine from the data
P4O6(s) + 2 O2(g) + 6 H2O(g) 4 H3PO4(s)
Assuming that all compounds are present in their most stable state at 25°C and 1 atm pressure?
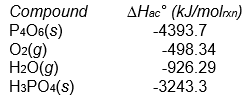
A) positive
B) negative
C) impossible to determine from the data

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
32
What is the absolute value of the enthalpy of reaction in the previous question?
A) less than 1000 kJ/molrxn
B) between 1000 and 1250 kJ/molrxn
C) between 1250 and 1500 kJ/molrxn
D) between 1500 and 1750 kJ/molrxn
E) more than 1750 kJ/molrxn
A) less than 1000 kJ/molrxn
B) between 1000 and 1250 kJ/molrxn
C) between 1250 and 1500 kJ/molrxn
D) between 1500 and 1750 kJ/molrxn
E) more than 1750 kJ/molrxn

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
33
Calculate the average Si-Br bond strength for an individual Si-Br bond in SiBr4. The Hac for SiBr4(g) is -1272 kJ/molrxn.
A) 3.66 kJ/mol
B) 254 kJ/mol
C) 318 kJ/mol
D) 636 kJ/mol
E) 1272 kJ/mol
A) 3.66 kJ/mol
B) 254 kJ/mol
C) 318 kJ/mol
D) 636 kJ/mol
E) 1272 kJ/mol

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
34
The disposable lighters that many smokers carry use butane as a fuel. Butane occurs as two isomers, n-butane
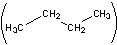
and isobutane
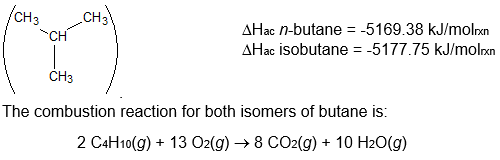
(I) Which form of butane will release the most heat when it is burned by the above combustion reaction? Explain your reasoning.
(II) Which form of butane will have the strongest bonds? Explain your reasoning.
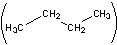
and isobutane

(I) Which form of butane will release the most heat when it is burned by the above combustion reaction? Explain your reasoning.
(II) Which form of butane will have the strongest bonds? Explain your reasoning.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
35
Use enthalpies of atom combination to calculate the change in enthalpy for
the reaction:
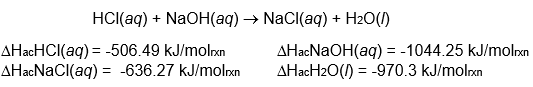
Is the reaction endothermic or exothermic?
the reaction:
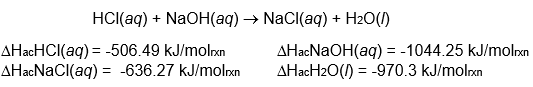
Is the reaction endothermic or exothermic?

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
36
The enthalpy of atom combination for CCl4(g) is -1306.3 kJ/molrxn. Calculate the average bond strength of the C-Cl bond.
A) 261.3 kJ/mol
B) 326.6 kJ/mol
C) 1306.3 kJ/mol
D) 3484.0 kJ/mol
E) 5225.2 kJ/mol
A) 261.3 kJ/mol
B) 326.6 kJ/mol
C) 1306.3 kJ/mol
D) 3484.0 kJ/mol
E) 5225.2 kJ/mol

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
37
Predict which of the following gases has the largest (most negative) enthalpy of atom combination?
A) CCl4
B) CBr4
C) CF4
D) CI4
E) They all have the same enthalpy of atom combination.
A) CCl4
B) CBr4
C) CF4
D) CI4
E) They all have the same enthalpy of atom combination.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following has the shortest carbon to halogen bond length?
A) CCl4
B) CBr4
C) CF4
D) CI4
E) All the bonds are the same length.
A) CCl4
B) CBr4
C) CF4
D) CI4
E) All the bonds are the same length.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
39
Arrange the following in order of increasing enthalpy of atom combination and decreasing bond length.
A) Cl2(g) > Br2(g) > F2(g) > I2(g)
B) F2(g) > Cl2(g) > Br2(g) > I2(g)
C) I2(g) > Br2(g) > Cl2(g) > F2(g)
D) F2(g) > Cl2(g) > I2(g) > Br2(g)
E) Cl2(g) > Br2(g) > I2(g) > Br2(g)
A) Cl2(g) > Br2(g) > F2(g) > I2(g)
B) F2(g) > Cl2(g) > Br2(g) > I2(g)
C) I2(g) > Br2(g) > Cl2(g) > F2(g)
D) F2(g) > Cl2(g) > I2(g) > Br2(g)
E) Cl2(g) > Br2(g) > I2(g) > Br2(g)

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
40
Arrange the following in order of increasing bond length and decreasing enthalpy of atom combination.
A) TeH4 > SiH4 > GeH4 > CH4
B) TeH4 > GeH4 > SiH4 > CH4
C) CH4 > SiH4 > GeH4 > TeH4
D) GeH4 > CH4 > TeH4 > SiH4
E) TeH4 > SiH4 > CH4 > GeH4
A) TeH4 > SiH4 > GeH4 > CH4
B) TeH4 > GeH4 > SiH4 > CH4
C) CH4 > SiH4 > GeH4 > TeH4
D) GeH4 > CH4 > TeH4 > SiH4
E) TeH4 > SiH4 > CH4 > GeH4

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
41
Use the following data
2 H2(g) + O2(g) 2 H2O(g) Hrxn° = -483.6 kJ/molrxn
2 H2(g) + O2(g) 2 H2O(l) Hrxn° = -571.6 kJ/molrxn
To calculate Hrxn° for the reaction
H2O(l) H2O(g)
A) -527.6 kJ/molrxn
B) -44.0 kJ/molrxn
C) 44.0 kJ/molrxn
D) 527.6 kJ/molrxn
E) none of the above
2 H2(g) + O2(g) 2 H2O(g) Hrxn° = -483.6 kJ/molrxn
2 H2(g) + O2(g) 2 H2O(l) Hrxn° = -571.6 kJ/molrxn
To calculate Hrxn° for the reaction
H2O(l) H2O(g)
A) -527.6 kJ/molrxn
B) -44.0 kJ/molrxn
C) 44.0 kJ/molrxn
D) 527.6 kJ/molrxn
E) none of the above

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
42
What is Hrxn° for the reaction: 2 CO(g) + O2(g) 2 CO2(g) if:
C(s) + 1/2 O2(g) CO(g) Hrxn° = -111 kJ/molrxn
C(s) + O2(g) CO2(g) Hrxn° = -393 kJ/molrxn
A) -564 kJ/molrxn
B) -282 kJ/molrxn
C) 171 kJ/molrxn
D) 282 kJ/molrxn
E) 564 kJ/molrxn
C(s) + 1/2 O2(g) CO(g) Hrxn° = -111 kJ/molrxn
C(s) + O2(g) CO2(g) Hrxn° = -393 kJ/molrxn
A) -564 kJ/molrxn
B) -282 kJ/molrxn
C) 171 kJ/molrxn
D) 282 kJ/molrxn
E) 564 kJ/molrxn

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
43
Calculate Hrxn° for the reaction: C(s) + 2 H2(g) CH4(g) from the following data:
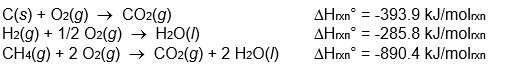
A) -1855.7 kJ/molrxn
B) -214.6 kJ/molrxn
C) -75.1 kJ/molrxn
D) 210.9 kJ/molrxn
E) 1569.9 kJ/molrxn
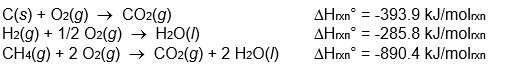
A) -1855.7 kJ/molrxn
B) -214.6 kJ/molrxn
C) -75.1 kJ/molrxn
D) 210.9 kJ/molrxn
E) 1569.9 kJ/molrxn

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
44
Given the following data
3 H2(g) + N2(g) 2 NH3(g) Hrxn° = -92.4 kJ/molrxn
2 H2(g) + O2(g) 2 H2O(l) Hrxn° = -571.7 kJ/molrxn
Calculate Hrxn° for the following reaction:
4 NH3(g) + 3 O2(g) 2 N2(g) + 6 H2O(l)
A) -1899.9 kJ/molrxn
B) -1715.1 kJ/molrxn
C) -1530.3 kJ/molrxn
D) -479.3 kJ/molrxn
E) 1530.3 kJ/molrxn
3 H2(g) + N2(g) 2 NH3(g) Hrxn° = -92.4 kJ/molrxn
2 H2(g) + O2(g) 2 H2O(l) Hrxn° = -571.7 kJ/molrxn
Calculate Hrxn° for the following reaction:
4 NH3(g) + 3 O2(g) 2 N2(g) + 6 H2O(l)
A) -1899.9 kJ/molrxn
B) -1715.1 kJ/molrxn
C) -1530.3 kJ/molrxn
D) -479.3 kJ/molrxn
E) 1530.3 kJ/molrxn

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
45
Hydrogen peroxide is a good oxidizing agent because it is a good source of molecular oxygen: 2 H2O2(aq) 2 H2O(l) + O2(g). Calculate Hrxn° for this reaction from the enthalpies of the following reactions.
2 H2(g) + O2(g) 2 H2O(l) Hrxn° = -571.66 kJ/molrxn
H2(g) + O2(g) H2O2(aq) Hrxn° = -187.8 kJ/molrxn
A) -759.5 kJ/molrxn
B) -383.9 kJ/molrxn
C) -196.0 kJ/molrxn
D) -98.0 kJ/molrxn
E) 196.0 kJ/molrxn
2 H2(g) + O2(g) 2 H2O(l) Hrxn° = -571.66 kJ/molrxn
H2(g) + O2(g) H2O2(aq) Hrxn° = -187.8 kJ/molrxn
A) -759.5 kJ/molrxn
B) -383.9 kJ/molrxn
C) -196.0 kJ/molrxn
D) -98.0 kJ/molrxn
E) 196.0 kJ/molrxn

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
46
Calculate Hrxn° for the reaction
N2(g) + O2(g) 2 NO(g)
From the enthalpies of the following reactions.
N2(g) + 2 O2(g) 2 NO2(g) Hrxn° = 66.4 kJ/molrxn
2 NO(g) + O2(g) 2 NO2(g) Hrxn° = -114.2 kJ/molrxn
A) -180.6 kJ/molrxn
B) -47.8 kJ/molrxn
C) 47.8 kJ/molrxn
D) 180.6 kJ/molrxn
E) Hrxn° is impossible to calculate from the information given
N2(g) + O2(g) 2 NO(g)
From the enthalpies of the following reactions.
N2(g) + 2 O2(g) 2 NO2(g) Hrxn° = 66.4 kJ/molrxn
2 NO(g) + O2(g) 2 NO2(g) Hrxn° = -114.2 kJ/molrxn
A) -180.6 kJ/molrxn
B) -47.8 kJ/molrxn
C) 47.8 kJ/molrxn
D) 180.6 kJ/molrxn
E) Hrxn° is impossible to calculate from the information given

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
47
Calculate the heat of combustion of propane, C3H8,
C3H8(g) + 5 O2(g) 3 CO2(g) + 4 H2O(g)
from the enthalpies of the following reactions.
3 C(s) + 4 H2(g) C3H8(g) Hrxn° = -103.85 kJ/molrxn
C(s) + O2(g) CO2(g) Hrxn° = -393.51 kJ/molrxn
H2(g) + 1/2 O2(g) H2O(g) Hrxn° = -241.83 kJ/molrxn
C3H8(g) + 5 O2(g) 3 CO2(g) + 4 H2O(g)
from the enthalpies of the following reactions.
3 C(s) + 4 H2(g) C3H8(g) Hrxn° = -103.85 kJ/molrxn
C(s) + O2(g) CO2(g) Hrxn° = -393.51 kJ/molrxn
H2(g) + 1/2 O2(g) H2O(g) Hrxn° = -241.83 kJ/molrxn

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
48
Calculate the heat required to transform 18 g of ice at 0°C to water vapor at 100°C using some or all of the data given below.
H2O(s) H2O(l) Hrxn° = 6.03 kJ/molrxn
H2O(l) H2O(g) Hrxn° = 40.67 kJ/molrxn
2 H2(g) + O2(g) 2 H2O(g) Hrxn° = -484 kJ/molrxn
H2O(liquid, 0°C) H2O(liquid, 100°C) Hrxn° = 7.53 kJ/molrxn
A) 40.67 kJ
B) 46.70 kJ
C) 54.23 kJ
D) 296 kJ
E) none of these
H2O(s) H2O(l) Hrxn° = 6.03 kJ/molrxn
H2O(l) H2O(g) Hrxn° = 40.67 kJ/molrxn
2 H2(g) + O2(g) 2 H2O(g) Hrxn° = -484 kJ/molrxn
H2O(liquid, 0°C) H2O(liquid, 100°C) Hrxn° = 7.53 kJ/molrxn
A) 40.67 kJ
B) 46.70 kJ
C) 54.23 kJ
D) 296 kJ
E) none of these

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
49
(Note that some of these are the same problems as found in the section headed Enthalpies of Atom Combination. However in this section all problems are worked using Enthalpies of Formation.)
-For which of the following substances is the enthalpy of formation, Hf S1° , equal to zero?
A) H2(l)
B) H2O(g)
C) O3(g)
D) F2(g)
E) Na(g)
-For which of the following substances is the enthalpy of formation, Hf S1° , equal to zero?
A) H2(l)
B) H2O(g)
C) O3(g)
D) F2(g)
E) Na(g)

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
50
(Note that some of these are the same problems as found in the section headed Enthalpies of Atom Combination. However in this section all problems are worked using Enthalpies of Formation.)
-Determine the change in enthalpy for the following reaction:
SnO2(s) + 2 H2(g) Sn(s) + 2 H2O(l)
Hf ° SnO2(s) = -580.7 kJ/molrxn Hf ° H2O(l) = -285.83 kJ/molrxn
A) -3088 kJ/molrxn
B) + 544 kJ/molrxn
C) -544 kJ/molrxn
D) + 9.0 kJ/molrxn
E) none of these
-Determine the change in enthalpy for the following reaction:
SnO2(s) + 2 H2(g) Sn(s) + 2 H2O(l)
Hf ° SnO2(s) = -580.7 kJ/molrxn Hf ° H2O(l) = -285.83 kJ/molrxn
A) -3088 kJ/molrxn
B) + 544 kJ/molrxn
C) -544 kJ/molrxn
D) + 9.0 kJ/molrxn
E) none of these

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
51
(Note that some of these are the same problems as found in the section headed Enthalpies of Atom Combination. However in this section all problems are worked using Enthalpies of Formation.)
-Use enthalpies of formation to calculate H ° for the following reaction.
C(s) + H2O(g) CO(g) + H2(g)
What would happen to the magnitude of H ° if the reaction consumed liquid water instead of gaseous water?

-Use enthalpies of formation to calculate H ° for the following reaction.
C(s) + H2O(g) CO(g) + H2(g)
What would happen to the magnitude of H ° if the reaction consumed liquid water instead of gaseous water?


Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
52
(Note that some of these are the same problems as found in the section headed Enthalpies of Atom Combination. However in this section all problems are worked using Enthalpies of Formation.)
-Calculate H ° for the following reaction from the data given below.
4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g)
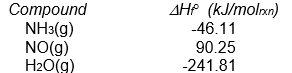
A) -105.5 kJ/molrxn
B) -905.4 kJ/molrxn
C) -1274.2 kJ/molrxn
D) -1996.2 kJ/molrxn
E) none of the above
-Calculate H ° for the following reaction from the data given below.
4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g)
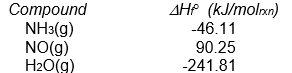
A) -105.5 kJ/molrxn
B) -905.4 kJ/molrxn
C) -1274.2 kJ/molrxn
D) -1996.2 kJ/molrxn
E) none of the above

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
53
(Note that some of these are the same problems as found in the section headed Enthalpies of Atom Combination. However in this section all problems are worked using Enthalpies of Formation.)
-Calculate H ° for the reaction
3 NO2(g) + H2O(l) 2 HNO3(aq) + NO(g)
From the following enthalpy of formation data.

A) less than -1000 kJ/molrxn
B) between -1000 and -750 kJ/molrxn
C) between -750 and -500 kJ/molrxn
D) between -500 and -250 kJ/molrxn
E) between -250 and 0 kJ/molrxn
-Calculate H ° for the reaction
3 NO2(g) + H2O(l) 2 HNO3(aq) + NO(g)
From the following enthalpy of formation data.

A) less than -1000 kJ/molrxn
B) between -1000 and -750 kJ/molrxn
C) between -750 and -500 kJ/molrxn
D) between -500 and -250 kJ/molrxn
E) between -250 and 0 kJ/molrxn

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
54
(Note that some of these are the same problems as found in the section headed Enthalpies of Atom Combination. However in this section all problems are worked using Enthalpies of Formation.)
-The following reaction occurs when sucrose (cane sugar) is metabolized by the body.
C12H22O11(s) + 12 O2(g) 12 CO2(g) + 11 H2O(l)
Given that H ° for this reaction is -5645 kJ/molrxn, what is the value of Hf ° for sucrose?
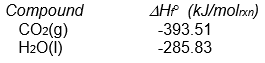
-The following reaction occurs when sucrose (cane sugar) is metabolized by the body.
C12H22O11(s) + 12 O2(g) 12 CO2(g) + 11 H2O(l)
Given that H ° for this reaction is -5645 kJ/molrxn, what is the value of Hf ° for sucrose?
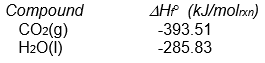

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
55
(Note that some of these are the same problems as found in the section headed Enthalpies of Atom Combination. However in this section all problems are worked using Enthalpies of Formation.)
-Both ethanol (CH3CH2OH) and methanol (CH3OH) have been considered as fuels for automobiles. Which is the better fuel, on a per gram basis, when burned with oxygen?

-Both ethanol (CH3CH2OH) and methanol (CH3OH) have been considered as fuels for automobiles. Which is the better fuel, on a per gram basis, when burned with oxygen?


Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
56
(Note that some of these are the same problems as found in the section headed Enthalpies of Atom Combination. However in this section all problems are worked using Enthalpies of Formation.)
-What is the sign of the enthalpy of reaction for the reaction P4O6(s) + 2 O2(g) + 6 H2O(g) 4 H3PO4(s)
Assuming that all compounds are present in their most stable state at 25°C and 1 atm pressure?
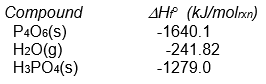
A) positive
B) negative
C) impossible to determine from the data
-What is the sign of the enthalpy of reaction for the reaction P4O6(s) + 2 O2(g) + 6 H2O(g) 4 H3PO4(s)
Assuming that all compounds are present in their most stable state at 25°C and 1 atm pressure?
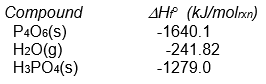
A) positive
B) negative
C) impossible to determine from the data

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
57
(Note that some of these are the same problems as found in the section headed Enthalpies of Atom Combination. However in this section all problems are worked using Enthalpies of Formation.)
-What is the magnitude of the enthalpy of reaction in the previous question?
A) less than 1000 kJ/molrxn
B) between 1000 and 1250 kJ/molrxn
C) between 1250 and 1500 kJ/molrxn
D) between 1500 and 1750 kJ/molrxn
E) more than 1750 kJ/molrxn
-What is the magnitude of the enthalpy of reaction in the previous question?
A) less than 1000 kJ/molrxn
B) between 1000 and 1250 kJ/molrxn
C) between 1250 and 1500 kJ/molrxn
D) between 1500 and 1750 kJ/molrxn
E) more than 1750 kJ/molrxn

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
58
(Note that some of these are the same problems as found in the section headed Enthalpies of Atom Combination. However in this section all problems are worked using Enthalpies of Formation.)
-For which of the following substances is the enthalpy of formation, Hf°,
Equal to zero?
A) H2(g)
B) H2O(g)
C) O3(g)
D) H(g)
E) Na(g)
-For which of the following substances is the enthalpy of formation, Hf°,
Equal to zero?
A) H2(g)
B) H2O(g)
C) O3(g)
D) H(g)
E) Na(g)

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
59
(Note that some of these are the same problems as found in the section headed Enthalpies of Atom Combination. However in this section all problems are worked using Enthalpies of Formation.)
-For which of the following substances is the enthalpy of formation, Hf S1° , equal to zero?
A) H2O(l)
B) H2O(s)
C) Cl(g)
D) P4(s)
E) CO2(g)
-For which of the following substances is the enthalpy of formation, Hf S1° , equal to zero?
A) H2O(l)
B) H2O(s)
C) Cl(g)
D) P4(s)
E) CO2(g)

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
60
The reaction below shows two isomers of benzene, a compound with the formula C6H6. The structure of benzene was a point of great controversy in the late 1800's and the two structures shown in the reaction were the chief contenders.
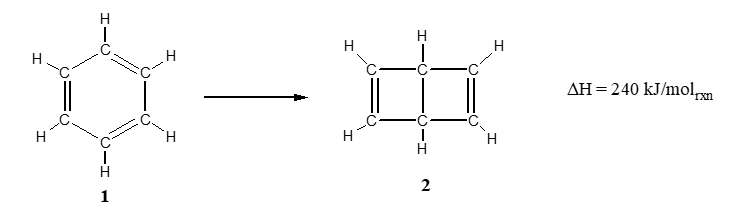
Which of the following statements is correct?
A) Isomer 1 is more stable than isomer 2
B) Isomer 2 is more stable than isomer 1
C) Isomer 2 has overall stronger bonds than isomer 1
D) Isomer 2 has stronger bonds and is more stable that isomer 1
E) Isomer 1 has weaker bonds and is more stable than isomer 2

Which of the following statements is correct?
A) Isomer 1 is more stable than isomer 2
B) Isomer 2 is more stable than isomer 1
C) Isomer 2 has overall stronger bonds than isomer 1
D) Isomer 2 has stronger bonds and is more stable that isomer 1
E) Isomer 1 has weaker bonds and is more stable than isomer 2

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
61
Retailers purchase gasoline by weight so they get a constant amount of gasoline per dollar (as long as the price is constant). We buy gasoline by volume. The enthalpy of combustion of 1.00 L of gasoline is -3.539 x 104 kJ at 15.6oC when gasoline has a density of 0.73722 g/mL. What would the enthalpy of combustion of 1.00 L of gasoline be at 32.2oC when gasoline has a density of 0.72210 g/mL?
A) -3.466 x 104 kJ
B) -3.539 x 104 kJ
C) -2.592 x 104 kJ
D) -2.373 x 104 kJ
E) None of the above
A) -3.466 x 104 kJ
B) -3.539 x 104 kJ
C) -2.592 x 104 kJ
D) -2.373 x 104 kJ
E) None of the above

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following is not true for an exothermic reaction?
A) Heat flows from the reaction system to the surroundings.
B) The temperature of the surroundings rise.
C) The enthalpy change for the reaction is negative.
D) The products have a higher energy than the reactants.
E) The bonds in the products are stronger than the bonds in the reactants.
A) Heat flows from the reaction system to the surroundings.
B) The temperature of the surroundings rise.
C) The enthalpy change for the reaction is negative.
D) The products have a higher energy than the reactants.
E) The bonds in the products are stronger than the bonds in the reactants.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
63
Write a balanced equation for the combustion of carbon disulfide to form carbon dioxide and sulfur dioxide, draw the Lewis structures of all reactants and products, and calculate Hrxn° for this reaction.
_____ CS2(g) + _____ O2(g) _____ CO2(g) + _____ SO2(g)
_____ CS2(g) + _____ O2(g) _____ CO2(g) + _____ SO2(g)

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
64
Use the following standard enthalpies of atom combination to
Hac° H2(g) = -435.30 kJ/molrxn Hac F2(g) = -157.98 kJ/molrxn
Hac° N2(g) = -945.41 kJ/molrxn Hac° C(s) = -716.68 kJ/molrxn
Hac° O2(g) = -498.34 kJ/molrxn Hac° NH3(g) = -1171.8 kJ/molrxn
Hac° H2O(g) = -926.3 kJ/molrxn Hac° HF(g) = -567.7 kJ/molrxn
-What is Hrxn° for the following reaction?
3 N(g) 3/2 N2(g)
A) +710 kJ/molrxn
B) -710 kJ/molrxn
C) +1418 kJ/molrxn
D) -1418 kJ/molrxn
E) -473 kJ/molrxn
Hac° H2(g) = -435.30 kJ/molrxn Hac F2(g) = -157.98 kJ/molrxn
Hac° N2(g) = -945.41 kJ/molrxn Hac° C(s) = -716.68 kJ/molrxn
Hac° O2(g) = -498.34 kJ/molrxn Hac° NH3(g) = -1171.8 kJ/molrxn
Hac° H2O(g) = -926.3 kJ/molrxn Hac° HF(g) = -567.7 kJ/molrxn
-What is Hrxn° for the following reaction?
3 N(g) 3/2 N2(g)
A) +710 kJ/molrxn
B) -710 kJ/molrxn
C) +1418 kJ/molrxn
D) -1418 kJ/molrxn
E) -473 kJ/molrxn

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
65
Use the following standard enthalpies of atom combination to
Hac° H2(g) = -435.30 kJ/molrxn Hac F2(g) = -157.98 kJ/molrxn
Hac° N2(g) = -945.41 kJ/molrxn Hac° C(s) = -716.68 kJ/molrxn
Hac° O2(g) = -498.34 kJ/molrxn Hac° NH3(g) = -1171.8 kJ/molrxn
Hac° H2O(g) = -926.3 kJ/molrxn Hac° HF(g) = -567.7 kJ/molrxn
-What is the bond energy of N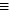 N?
N?
A) 237 kJ/mol
B) 158 kJ/mol
C) 473 kJ/mol
D) 946 kJ/mol
E) 1892 kJ/mol
Hac° H2(g) = -435.30 kJ/molrxn Hac F2(g) = -157.98 kJ/molrxn
Hac° N2(g) = -945.41 kJ/molrxn Hac° C(s) = -716.68 kJ/molrxn
Hac° O2(g) = -498.34 kJ/molrxn Hac° NH3(g) = -1171.8 kJ/molrxn
Hac° H2O(g) = -926.3 kJ/molrxn Hac° HF(g) = -567.7 kJ/molrxn
-What is the bond energy of N
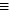 N?
N?A) 237 kJ/mol
B) 158 kJ/mol
C) 473 kJ/mol
D) 946 kJ/mol
E) 1892 kJ/mol

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
66
Use the following standard enthalpies of atom combination to
Hac° H2(g) = -435.30 kJ/molrxn Hac F2(g) = -157.98 kJ/molrxn
Hac° N2(g) = -945.41 kJ/molrxn Hac° C(s) = -716.68 kJ/molrxn
Hac° O2(g) = -498.34 kJ/molrxn Hac° NH3(g) = -1171.8 kJ/molrxn
Hac° H2O(g) = -926.3 kJ/molrxn Hac° HF(g) = -567.7 kJ/molrxn
-What is the Hrxn° for the following reaction as written?
4 NH3(g) + 3 O2(g) 2 N2(g) + 6 H2O(g)
A) -1266 kJ/molrxn
B) -1184 kJ/molrxn
C) -196 kJ/molrxn
D) +767 kJ/molrxn
E) none of the above
Hac° H2(g) = -435.30 kJ/molrxn Hac F2(g) = -157.98 kJ/molrxn
Hac° N2(g) = -945.41 kJ/molrxn Hac° C(s) = -716.68 kJ/molrxn
Hac° O2(g) = -498.34 kJ/molrxn Hac° NH3(g) = -1171.8 kJ/molrxn
Hac° H2O(g) = -926.3 kJ/molrxn Hac° HF(g) = -567.7 kJ/molrxn
-What is the Hrxn° for the following reaction as written?
4 NH3(g) + 3 O2(g) 2 N2(g) + 6 H2O(g)
A) -1266 kJ/molrxn
B) -1184 kJ/molrxn
C) -196 kJ/molrxn
D) +767 kJ/molrxn
E) none of the above

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
67
Use the following standard enthalpies of atom combination to
Hac° H2(g) = -435.30 kJ/molrxn Hac F2(g) = -157.98 kJ/molrxn
Hac° N2(g) = -945.41 kJ/molrxn Hac° C(s) = -716.68 kJ/molrxn
Hac° O2(g) = -498.34 kJ/molrxn Hac° NH3(g) = -1171.8 kJ/molrxn
Hac° H2O(g) = -926.3 kJ/molrxn Hac° HF(g) = -567.7 kJ/molrxn
-What is the bond energy, H2N-H, for one of the N-H bonds in NH3(g)?
A) 391 kJ/mol
B) 645 kJ/mol
C) 737 kJ/mol
D) 846 kJ/mol
E) 1173 kJ/moI
Hac° H2(g) = -435.30 kJ/molrxn Hac F2(g) = -157.98 kJ/molrxn
Hac° N2(g) = -945.41 kJ/molrxn Hac° C(s) = -716.68 kJ/molrxn
Hac° O2(g) = -498.34 kJ/molrxn Hac° NH3(g) = -1171.8 kJ/molrxn
Hac° H2O(g) = -926.3 kJ/molrxn Hac° HF(g) = -567.7 kJ/molrxn
-What is the bond energy, H2N-H, for one of the N-H bonds in NH3(g)?
A) 391 kJ/mol
B) 645 kJ/mol
C) 737 kJ/mol
D) 846 kJ/mol
E) 1173 kJ/moI

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
68
Use the following standard enthalpies of atom combination to
Hac° H2(g) = -435.30 kJ/molrxn Hac F2(g) = -157.98 kJ/molrxn
Hac° N2(g) = -945.41 kJ/molrxn Hac° C(s) = -716.68 kJ/molrxn
Hac° O2(g) = -498.34 kJ/molrxn Hac° NH3(g) = -1171.8 kJ/molrxn
Hac° H2O(g) = -926.3 kJ/molrxn Hac° HF(g) = -567.7 kJ/molrxn
-How much heat is absorbed or evolved when just enough H2(g) reacts with just enough F2(g) to produce 0.200 mol of HF(g)?
A) 54 kJ of heat is absorbed
B) 54 kJ of heat is evolved
C) 108 kJ of heat is absorbed
D) 108 kJ of heat is evolved
E) 216 kJ of heat is absorbed
Hac° H2(g) = -435.30 kJ/molrxn Hac F2(g) = -157.98 kJ/molrxn
Hac° N2(g) = -945.41 kJ/molrxn Hac° C(s) = -716.68 kJ/molrxn
Hac° O2(g) = -498.34 kJ/molrxn Hac° NH3(g) = -1171.8 kJ/molrxn
Hac° H2O(g) = -926.3 kJ/molrxn Hac° HF(g) = -567.7 kJ/molrxn
-How much heat is absorbed or evolved when just enough H2(g) reacts with just enough F2(g) to produce 0.200 mol of HF(g)?
A) 54 kJ of heat is absorbed
B) 54 kJ of heat is evolved
C) 108 kJ of heat is absorbed
D) 108 kJ of heat is evolved
E) 216 kJ of heat is absorbed

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck
69
Consider the following data for heats of atom combination of three substances in the liquid and gaseous states.
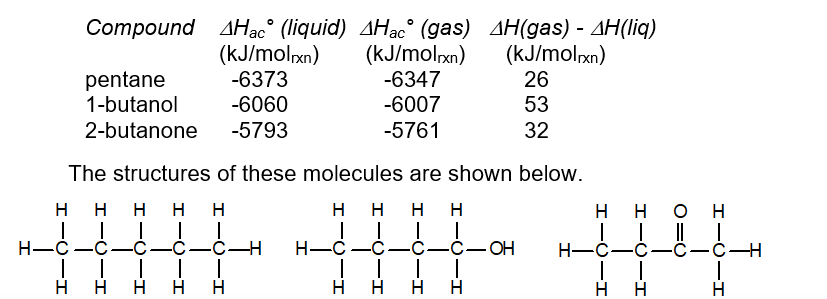
Explain the relative magnitudes of the differences between the enthalpies of atom combination of the liquid and gaseous states of these three compounds.

Explain the relative magnitudes of the differences between the enthalpies of atom combination of the liquid and gaseous states of these three compounds.

Unlock Deck
Unlock for access to all 69 flashcards in this deck.
Unlock Deck
k this deck


