Deck 5: Ionic and Metallic Bonds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/80
Play
Full screen (f)
Deck 5: Ionic and Metallic Bonds
1
Which of the following sets of elements is arranged in order of increasing
Metallic character?
A) Al < Cs < Sr
B) Ba < Sr < Ca
C) Na < Mg < Al
D) Al < Ca < Ba
E) none of these
Metallic character?
A) Al < Cs < Sr
B) Ba < Sr < Ca
C) Na < Mg < Al
D) Al < Ca < Ba
E) none of these
Al < Ca < Ba
2
Which of the following metals is the most "active," the metal which should react most rapidly with oxygen or water vapor in the atmosphere?
A) Be
B) Ca
C) Ba
D) Al
E) Ga
A) Be
B) Ca
C) Ba
D) Al
E) Ga
Ba
3
Which of the following metals would be the most reactive towards air and water?
A) Ca
B) Al
C) Ag
D) Sn
E) Pb
A) Ca
B) Al
C) Ag
D) Sn
E) Pb
Ca
4
Predict the formula of the neutral ionic compound formed from K+ and S2-.
A) KS
B) K2S
C) KS3
D) K2S3
E) none of these
A) KS
B) K2S
C) KS3
D) K2S3
E) none of these

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
5
Predict the formula of the neutral ionic compound formed from Mg2+ and PO43-.
A) MgPO4
B) Mg3PO4
C) Mg(PO4)2
D) Mg3(PO4)2
E) none of these
A) MgPO4
B) Mg3PO4
C) Mg(PO4)2
D) Mg3(PO4)2
E) none of these

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
6
Predict the formula of the electrically neutral compound formed from the
Following ions: Ca2+ and PO43-.
A) CaPO4
B) Ca2PO4
C) Ca3(PO4)2
D) Ca2(PO4)3
E) none of these
Following ions: Ca2+ and PO43-.
A) CaPO4
B) Ca2PO4
C) Ca3(PO4)2
D) Ca2(PO4)3
E) none of these

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
7
Predict the formula of the electrically neutral compound formed from the following ions: K+ and CrO42-.
A) KCrO4
B) K(CrO4)2
C) K3(CrO4)2
D) K2(CrO4)3
E) none of these
A) KCrO4
B) K(CrO4)2
C) K3(CrO4)2
D) K2(CrO4)3
E) none of these

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
8
Predict the formula of the ionic compound formed between an aluminum cation, Al3+, and a carbonate polyatomic anion.
A) AlCO3
B) Al3CO3
C) Al2CO3
D) Al3(CO3)2
E) Al2(CO3)3
A) AlCO3
B) Al3CO3
C) Al2CO3
D) Al3(CO3)2
E) Al2(CO3)3

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
9
A mineral called montmorillonite will catalyze some interesting reactions. The formula of one type of montmorillonite is NaCa4(Si2O5)4F. If Na has a charge of +1, Ca has a charge of +2, and F has a charge of -1, what is the charge on each Si2O5 complex ion?
A) +2
B) +3
C) -2
D) +4
E) -4
A) +2
B) +3
C) -2
D) +4
E) -4

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
10
Aluminum chlorhydrate is added to antiperspirants to stop people from sweating. If this compound contains neutral H2O molecules and Al3+, OH-, and Cl- ions, and the formula of this compound is Alx(OH)5Cl • 2 H2O, what is the value of x in this formula?
A) 0.5
B) 1
C) 1.5
D) 2
E) 3
A) 0.5
B) 1
C) 1.5
D) 2
E) 3

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
11
Predict the formulas for neutral compounds containing the following pairs of ions.
A) H+ and O22-
B) Zn2+ and PO43-
C) K+ and [PtCl6]2-
A) H+ and O22-
B) Zn2+ and PO43-
C) K+ and [PtCl6]2-

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
12
What is the formula of the neutral compound which might be formed from P5+ and S2-?
A) PS
B) PS2
C) PS3
D) P2S5
E) P5S2
A) PS
B) PS2
C) PS3
D) P2S5
E) P5S2

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
13
If the formula for aluminum oxide is Al2O3, what would be the formulas for
aluminum fluoride and aluminum sulfide?
aluminum fluoride and aluminum sulfide?

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
14
What is the charge on the [Co(NO2)6]x ion if this complex ion is formed by combining Co3+ and NO2- ions?
A) +3
B) 0
C) -3
D) -6
E) -12
A) +3
B) 0
C) -3
D) -6
E) -12

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
15
A main-group reacts with hydrogen and oxygen to form compounds with the formulas XH4 and XO2 (X represents the metal). In which group of the periodic table does this element belong?
A) IA
B) IIA
C) IIIA
D) IVA
E) none of these
A) IA
B) IIA
C) IIIA
D) IVA
E) none of these

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
16
What is the most likely charge for a calcium ion?
A) -2
B) -1
C) 0
D) +1
E) +2
A) -2
B) -1
C) 0
D) +1
E) +2

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following elements would be the most likely to form an oxide with the formula XO and a hydride with the formula XH2 (X represents the unknown element)?
A) Na
B) Mg
C) Al
D) Si
E) P
A) Na
B) Mg
C) Al
D) Si
E) P

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
18
An element, X, forms the ionic compound CaX. X is a member of:
A) Group IA
B) Group IIA
C) Group IVA
D) Group VIA
E) Group VIIA
A) Group IA
B) Group IIA
C) Group IVA
D) Group VIA
E) Group VIIA

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
19
Magnesium metal reacts with hydrogen gas to form a white solid

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
20
Which element is hydrogen most like to combine with to form a hydride?
A) C
B) S
C) N
D) Na
E) O
A) C
B) S
C) N
D) Na
E) O

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
21
What is the most likely electronic configuration of the Co2+ ion?
A) [Ar]3d7
B) [Ar]4s23d7
C) [Ar]4s23d5
D) [Kr]5s24d7
E) None of the above
A) [Ar]3d7
B) [Ar]4s23d7
C) [Ar]4s23d5
D) [Kr]5s24d7
E) None of the above

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
22
What is the most likely electronic configuration of the Ru2+ ion?
A) [Kr]4d6
B) [Kr]5s24d6
C) [Kr]5s24d4
D) [Ar]4s23d6
E) None of the above
A) [Kr]4d6
B) [Kr]5s24d6
C) [Kr]5s24d4
D) [Ar]4s23d6
E) None of the above

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
23
An element reacts with hydrogen and oxygen to form ionic compounds with the formulas MH4 and MO2. In which group of the periodic table does this element, M, belong?
A) IIA
B) IVA
C) VA
D) VIA
E) VIIA
A) IIA
B) IVA
C) VA
D) VIA
E) VIIA

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
24
What group of metals react with sulfur to form M2S3 sulfides, reacts with fluorine to form MF3 fluorides, and react with acid to form M3+ ions and H2 gas?
A) IA
B) IIA
C) IIIA
D) IVA
E) VA
A) IA
B) IIA
C) IIIA
D) IVA
E) VA

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following elements would be the most likely to react with hydrogen to form a compound with the formula XH (X represents the metal)?
A) Na
B) Mg
C) Al
D) Si
E) P
A) Na
B) Mg
C) Al
D) Si
E) P

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
26
The two most common ions of copper are Cu1+ and Cu2+. Give the most likely electron configuration of each of these ions.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
27
Which ionic compound contains the superoxide ion?
A) CaO2
B) LiO2
C) Na2O2
D) H2O2
E) BaO
A) CaO2
B) LiO2
C) Na2O2
D) H2O2
E) BaO

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
28
Which ionic compound contains the peroxide ion?
A) CaO
B) LiO2
C) Na2O2
D) Na2O
E) None of the above
A) CaO
B) LiO2
C) Na2O2
D) Na2O
E) None of the above

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is the most likely product of the reaction between magnesium metal and nitrogen?
A) MgN
B) Mg2N
C) MgN2
D) Mg2N3
E) Mg3N2
A) MgN
B) Mg2N
C) MgN2
D) Mg2N3
E) Mg3N2

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
30
Use the positions of gallium and oxygen in the periodic table to predict the
Formula for gallium oxide.
A) GaO
B) GaO2
C) GaO3
D) Ga2O
E) Ga2O3
Formula for gallium oxide.
A) GaO
B) GaO2
C) GaO3
D) Ga2O
E) Ga2O3

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
31
Predict the product of the following reaction: Sr(s) + P4(s).
A) SrP
B) SrP2
C) SrP3
D) Sr2P3
E) Sr3P2
A) SrP
B) SrP2
C) SrP3
D) Sr2P3
E) Sr3P2

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
32
What would be the product of the reaction between aluminum metal and sulfur?
A) AlS
B) Al2S
C) AlS2
D) AlS3
E) Al2S3
A) AlS
B) Al2S
C) AlS2
D) AlS3
E) Al2S3

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
33
What compound is formed by the reaction of Na with P4?
A) NaP3
B) Na3P
C) NaP2
D) Na2P
E) NaP
A) NaP3
B) Na3P
C) NaP2
D) Na2P
E) NaP

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
34
Use the positions of silicon and fluorine in the periodic table to predict the most likely product of the reaction between silicon and fluorine.
A) SiF
B) SiF2
C) SiF4
D) Si2F
E) Si3F4
A) SiF
B) SiF2
C) SiF4
D) Si2F
E) Si3F4

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
35
Predict the product of the reaction between barium and nitrogen.
A) BaN
B) BaN2
C) Ba2N
D) BaN3
E) none of these
A) BaN
B) BaN2
C) Ba2N
D) BaN3
E) none of these

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
36
An element X forms a compound with oxygen, X2O3, and with hydrogen, XH3. The element X must be in which group in the periodic table?
A) IA
B) IIA
C) IIIA
D) IVA
E) VIA
A) IA
B) IIA
C) IIIA
D) IVA
E) VIA

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
37
In Chapter 4 we were able to determine the geometric shape and resulting polarity of individual covalent molecules. In Chapter 5 we did not do this for ionic and metallic compounds. Why not?
A) Ionic and metallic compounds exist as three dimensional networks not as individual molecules.
B) The electrons in ionic compounds are transferred rather than being shared as in covalent compounds.
C) The difference in electronegativities of the bonded atoms is usually greater in ionic than in covalent and metallic compounds.
D) Covalent molecules are much larger than ionic compounds.
E) all of these
A) Ionic and metallic compounds exist as three dimensional networks not as individual molecules.
B) The electrons in ionic compounds are transferred rather than being shared as in covalent compounds.
C) The difference in electronegativities of the bonded atoms is usually greater in ionic than in covalent and metallic compounds.
D) Covalent molecules are much larger than ionic compounds.
E) all of these

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
38
If the difference in electronegativity between two elements in a compound is very small (less then 0.2), can you safely conclude that the compound formed from the two elements is covalent?
A) Yes, because a small difference in electronegativity always indicates that a compound is covalent.
B) No, because covalent compounds are formed between two elements with a large difference in electronegativity.
C) Electronegativity has nothing to do with whether a bond is covalent or not.
D) No, because a metallic compound could also be formed between two elements with a small difference in electronegativity.
E) None of the above are correct.
A) Yes, because a small difference in electronegativity always indicates that a compound is covalent.
B) No, because covalent compounds are formed between two elements with a large difference in electronegativity.
C) Electronegativity has nothing to do with whether a bond is covalent or not.
D) No, because a metallic compound could also be formed between two elements with a small difference in electronegativity.
E) None of the above are correct.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
39
Compare and contrast (what is the same and what is different) the distribution of electrons in covalent, ionic and metallic bonds.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
40
The bond-type triangle can be used for
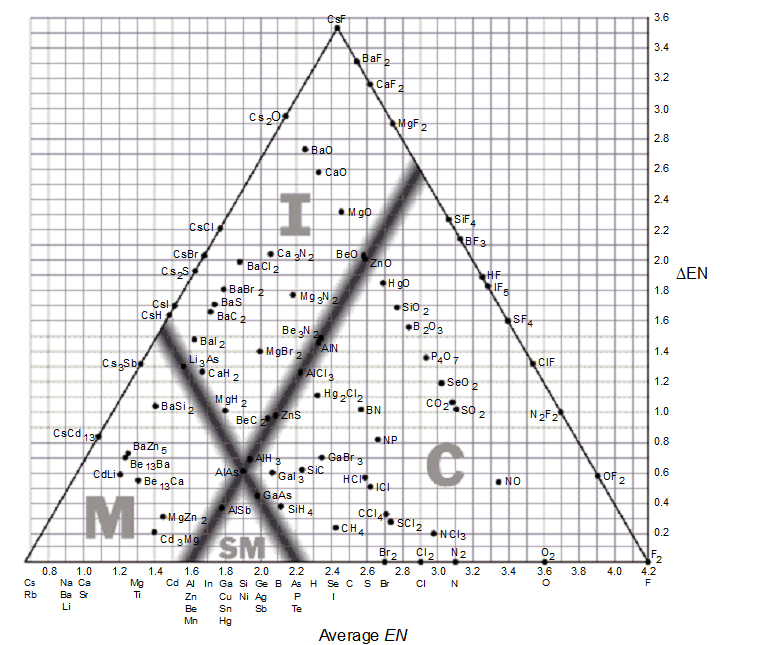
-Based on the bond-type triangle, which of these covalent compounds has the greatest ionic character?
A) NCl3
B) SCl2
C) ICl
D) HCl
E) SiC

-Based on the bond-type triangle, which of these covalent compounds has the greatest ionic character?
A) NCl3
B) SCl2
C) ICl
D) HCl
E) SiC

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
41
The bond-type triangle can be used for

-Using a bond-type triangle, predict which of the following compounds would have approximately 50% ionic bonding and 50% covalent bonding.
A) AlCl3
B) Li3As
C) AlAs
D) BN
E) none of these

-Using a bond-type triangle, predict which of the following compounds would have approximately 50% ionic bonding and 50% covalent bonding.
A) AlCl3
B) Li3As
C) AlAs
D) BN
E) none of these

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
42
The bond-type triangle can be used for

-Describe what happens to the distribution of electrons in the bond between two elements as you move from left to right on a bond-type triangle. Describe what happens to the distribution of the electrons in a bond between two elements as you move from the bottom to the top of a bond-type triangle.

-Describe what happens to the distribution of electrons in the bond between two elements as you move from left to right on a bond-type triangle. Describe what happens to the distribution of the electrons in a bond between two elements as you move from the bottom to the top of a bond-type triangle.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
43
The bond-type triangle can be used for

-Use a bond-type triangle to predict the correct arrangement of the following compounds in order of increasing covalent character.
Mg Zn2, CCl4, SiH4
A) Mg Zn2 < CCl4 < SiH4
B) CCl4 < Mg Zn2 < SiH4
C) CCl4 < SiH4 < Mg Zn2
D) MgZn2 < SiH4 < CCl4
E) none of these

-Use a bond-type triangle to predict the correct arrangement of the following compounds in order of increasing covalent character.
Mg Zn2, CCl4, SiH4
A) Mg Zn2 < CCl4 < SiH4
B) CCl4 < Mg Zn2 < SiH4
C) CCl4 < SiH4 < Mg Zn2
D) MgZn2 < SiH4 < CCl4
E) none of these

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
44
The bond-type triangle can be used for

-Use a bond-type triangle to describe the bonding in CaH2.
A) pure ionic
B) pure covalent
C) pure metallic
D) significant amount of both ionic and covalent character
E) significant amount of both ionic and metallic character

-Use a bond-type triangle to describe the bonding in CaH2.
A) pure ionic
B) pure covalent
C) pure metallic
D) significant amount of both ionic and covalent character
E) significant amount of both ionic and metallic character

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
45
The bond-type triangle can be used for

-As you move from the bottom to the top on a bond-type triangle, _________.
A) the solids can increasingly be described as an array of cations in a sea of electrons
B) compounds exist mostly as gases
C) the solid compounds become more conductive
D) the electron density in a bond is increasingly shared between two atoms, rather than being transferred
E) the electrons in a bond are transferred from one atom to another, rather than being shared

-As you move from the bottom to the top on a bond-type triangle, _________.
A) the solids can increasingly be described as an array of cations in a sea of electrons
B) compounds exist mostly as gases
C) the solid compounds become more conductive
D) the electron density in a bond is increasingly shared between two atoms, rather than being transferred
E) the electrons in a bond are transferred from one atom to another, rather than being shared

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
46
The bond-type triangle can be used for

-According to the supplied bond-type triangle, which of the following compounds is most likely to conduct electricity as a solid?
A) SO2
B) MgBr2
C) CdLi
D) NCl3
E) N2F2

-According to the supplied bond-type triangle, which of the following compounds is most likely to conduct electricity as a solid?
A) SO2
B) MgBr2
C) CdLi
D) NCl3
E) N2F2

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
47
The bond-type triangle can be used for

-Based on the given bond-type triangle, what is the highest possible average electronegativity a compound can have and still retain some metallic character?
A) Approximately 2.9
B) Approximately 2.7
C) Approximately 1.9
D) Approximately 1.4
E) A compound can have metallic character at any average electronegativity.

-Based on the given bond-type triangle, what is the highest possible average electronegativity a compound can have and still retain some metallic character?
A) Approximately 2.9
B) Approximately 2.7
C) Approximately 1.9
D) Approximately 1.4
E) A compound can have metallic character at any average electronegativity.

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
48
Determine the oxidation number of sulfur in potassium sulfate, K2SO4.
A) +2
B) +4
C) +6
D) +8
E) none of these
A) +2
B) +4
C) +6
D) +8
E) none of these

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
49
Determine the oxidation number of the carbon in bold in the following compound: CH3CCl3.
A) 0
B) -3
C) +1
D) +3
E) none of these
A) 0
B) -3
C) +1
D) +3
E) none of these

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
50
Determine the oxidation number of sulfur in sulfurous acid, H2SO3.
A) +6
B) +4
C) +2
D) -2
E) none of these
A) +6
B) +4
C) +2
D) -2
E) none of these

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
51
Determine the oxidation number of the central carbon atom in the following compound.
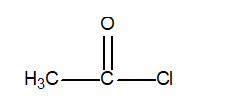
A) +2
B) -2
C) +4
D) -4
E) none of these

A) +2
B) -2
C) +4
D) -4
E) none of these

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
52
Determine the oxidation number of chlorine in NaClO3.
A) -1
B) +1
C) +3
D) +7
E) none of these
A) -1
B) +1
C) +3
D) +7
E) none of these

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
53
Determine the oxidation number of the second (bolded) carbon in CH3CH2NH2.
A) -1
B) +1
C) +3
D) -3
E) none of these
A) -1
B) +1
C) +3
D) -3
E) none of these

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following compounds has sulfur in a +6 oxidation state?
A) SO2
B) H2S
C) H2SO3
D) H2SO4
E) H2S2O3
A) SO2
B) H2S
C) H2SO3
D) H2SO4
E) H2S2O3

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
55
What is the maximum possible oxidation state of a P atom?
A) +2
B) +3
C) +4
D) +5
E) none of the above
A) +2
B) +3
C) +4
D) +5
E) none of the above

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
56
What is the oxidation state of the Mo atom in Li2MoO4?
A) 0
B) +2
C) +4
D) +5
E) +6
A) 0
B) +2
C) +4
D) +5
E) +6

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
57
All but one of the following species contains nitrogen in the same oxidation state. Which one is different?
A) HNO2
B) NH2-
C) NH3
D) NH4Cl
E) Na3N
A) HNO2
B) NH2-
C) NH3
D) NH4Cl
E) Na3N

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
58
Which compounds contain hydrogen in a negative oxidation state?
(I) H2S (II) PH4+ (III) LiAlH4 (IV) CH4 (V) CaH2
A) I, II and IV
B) II, III and IV
C) III and IV
D) III and V
E) none of these contain hydrogen in a negative oxidation state
(I) H2S (II) PH4+ (III) LiAlH4 (IV) CH4 (V) CaH2
A) I, II and IV
B) II, III and IV
C) III and IV
D) III and V
E) none of these contain hydrogen in a negative oxidation state

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
59
Honda and Toyota both sell hybrid cars that have engines that can run on either gasoline or electricity stored in a battery. The advantage of hybrid cars is simple: Totally electric cars need batteries that weigh almost as much as the vehicle! It isn't surprising that a high priority is the search for better batteries. Barium ferrate (BaFeO4) is being tested for use in batteries. Use the positions of barium and oxygen in the periodic table to predict the oxidation state of the iron atom in this compound.
A) +2
B) +3
C) +4
D) +6
E) +8
A) +2
B) +3
C) +4
D) +6
E) +8

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
60
Lithium ion batteries are high energy density 3.6 V batteries that use a
Lithium salt such as LiCoO2 or LiPF6 for the positive electrode, or cathode. Use the positions of lithium and fluorine in the periodic table to predict the oxidation state of the phosphorus atom in LiPF6.
A) +1
B) +2
C) +3
D) +4
E) +5
Lithium salt such as LiCoO2 or LiPF6 for the positive electrode, or cathode. Use the positions of lithium and fluorine in the periodic table to predict the oxidation state of the phosphorus atom in LiPF6.
A) +1
B) +2
C) +3
D) +4
E) +5

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
61
In the following redox reaction which element is being oxidized?
Cu(s) + 4 HNO3(aq) Cu(NO3)2 (aq) + 2 NO2
A) Cu
B) H
C) N
D) O
E) none of these (g) + 2 H2O(l)
Cu(s) + 4 HNO3(aq) Cu(NO3)2 (aq) + 2 NO2
A) Cu
B) H
C) N
D) O
E) none of these (g) + 2 H2O(l)

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
62
Which one of the following is an oxidation-reduction reaction?
A) CaCO3 (s) CaO (s) + CO2
B) CaO (s) + 2 HCl (aq) CaCl2 (aq) + H2O (l)
C) 2 KNO3 (s) 2 KNO2 (s) + O2 (g)
D) HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l)
E) none of these
A) CaCO3 (s) CaO (s) + CO2
B) CaO (s) + 2 HCl (aq) CaCl2 (aq) + H2O (l)
C) 2 KNO3 (s) 2 KNO2 (s) + O2 (g)
D) HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l)
E) none of these

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
63
In the following oxidation-reduction reaction, which of the following statements is true?
Sr(s) + 2 H2O(l) Sr2+(aq) + 2 OH-(aq) + H2(g)
A) H2O is oxidized
B) Sr acts as the oxidizing agent
C) H2 is reduced
D) H2O is the oxidizing agent
E) more than one of the above are true
Sr(s) + 2 H2O(l) Sr2+(aq) + 2 OH-(aq) + H2(g)
A) H2O is oxidized
B) Sr acts as the oxidizing agent
C) H2 is reduced
D) H2O is the oxidizing agent
E) more than one of the above are true

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
64
Phosphorus is reduced in which of the following reactions?
A) P4(s) + 5 O2(g) P4O10(s)
B) P4(s) + 3 O2(g) P4O6(s)
C) P4(s) + 6 F2(g) 4 PF3(g)
D) P4(s) + 6 Ca(s) 2 Ca3P2(s) + H2O(l)
E) P4(s) + 6 Cl2(g) 4 H3PO3(aq) + 12 HCl(aq)
A) P4(s) + 5 O2(g) P4O10(s)
B) P4(s) + 3 O2(g) P4O6(s)
C) P4(s) + 6 F2(g) 4 PF3(g)
D) P4(s) + 6 Ca(s) 2 Ca3P2(s) + H2O(l)
E) P4(s) + 6 Cl2(g) 4 H3PO3(aq) + 12 HCl(aq)

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
65
Which isn't an oxidation-reduction reaction?
A)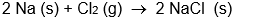
B)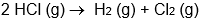
C)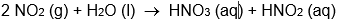
D)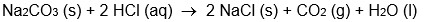
E) all of these are oxidation-reduction reactions
A)
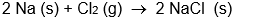
B)
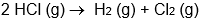
C)
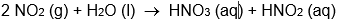
D)
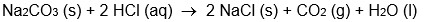
E) all of these are oxidation-reduction reactions

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
66
Identify the reducing agent and oxidizing agent in the reaction:
H2O2(aq) + 2 HI(aq) 2 H2O(l) + I2(s)
H2O2(aq) + 2 HI(aq) 2 H2O(l) + I2(s)

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
67
The name for Li3N is:
A) lithium nitride
B) lithium(II) nitride
C) lithium(III) nitride
D) lithium trinitride
E) trilithium nitride
A) lithium nitride
B) lithium(II) nitride
C) lithium(III) nitride
D) lithium trinitride
E) trilithium nitride

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following formula/name combinations is incorrect?
A) H2SO3/sulfurous acid
B) HCO3-/hydrogen carbonate ion
C) HBrO3/bromic acid
D) ClO4-/hypochlorate ion
E) CuSO4/copper(II) sulfate
A) H2SO3/sulfurous acid
B) HCO3-/hydrogen carbonate ion
C) HBrO3/bromic acid
D) ClO4-/hypochlorate ion
E) CuSO4/copper(II) sulfate

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
69
Name this compound: CoSO3
A) copper persulfate
B) cobalt(II) sulfite
C) cupric sulfate
D) cobalt (II) sulfide
E) cupric sulfite
A) copper persulfate
B) cobalt(II) sulfite
C) cupric sulfate
D) cobalt (II) sulfide
E) cupric sulfite

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
70
Name this compound: SrSO4
A) strontium sulfide
B) sodium sulfite
C) strontium persulfate
D) sodium sulfurtetroxide
E) strontium sulfate
A) strontium sulfide
B) sodium sulfite
C) strontium persulfate
D) sodium sulfurtetroxide
E) strontium sulfate

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
71
Give the chemical formula for barium chromate.
A) Ba2CrO4
B) Ba2CrO3
C) BaCr
D) BaCrO4
E) BCrO3
A) Ba2CrO4
B) Ba2CrO3
C) BaCr
D) BaCrO4
E) BCrO3

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
72
Give the chemical formula for ammonium bromide.
A) NBr3
B) NH4Br
C) AmBr2
D) NH4Br2
E) NH4Br3
A) NBr3
B) NH4Br
C) AmBr2
D) NH4Br2
E) NH4Br3

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
73
Give the name for the compound Fe2O3.
A) diiron trioxide
B) iron (III) oxide
C) iron (II) trioxide
D) iron oxide
E) iron trioxide
A) diiron trioxide
B) iron (III) oxide
C) iron (II) trioxide
D) iron oxide
E) iron trioxide

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
74
Give the chemical formula for perchloric acid.
A) HClO3
B) HCl
C) NaHCO3
D) HClO4
E) H2ClO3
A) HClO3
B) HCl
C) NaHCO3
D) HClO4
E) H2ClO3

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
75
Give the name for the compound ZnSO3.
A) zinc persulfate
B) zinc sulfur trioxide
C) zinc (II) sulfate
D) zinc (I) sulfide
E) zinc sulfite
A) zinc persulfate
B) zinc sulfur trioxide
C) zinc (II) sulfate
D) zinc (I) sulfide
E) zinc sulfite

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
76
Give the chemical formula for ammonium cyanide.
A) HCN
B) NH4CN
C) NH2C
D) CH3CN
E) AmCN
A) HCN
B) NH4CN
C) NH2C
D) CH3CN
E) AmCN

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
77
Give the name for the compound Cl2O.
A) dichoro oxide
B) chlorine oxide
C) dichlorine monoxide
D) chlorite
E) chlorine dioxide
A) dichoro oxide
B) chlorine oxide
C) dichlorine monoxide
D) chlorite
E) chlorine dioxide

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
78
Give the chemical formula for hyposulfurous acid.
A) H2SO2
B) H2S
C) NaHSO3
D) HSO3
E) H2SO4
A) H2SO2
B) H2S
C) NaHSO3
D) HSO3
E) H2SO4

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
79
Give the name for the compound HNO2.
A) hydrogen nitride
B) pernitric acid
C) nitrous acid
D) hydrogen nitrogen dioxide
E) nitric acid
A) hydrogen nitride
B) pernitric acid
C) nitrous acid
D) hydrogen nitrogen dioxide
E) nitric acid

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck
80
Give the chemical formula for copper(I)chloride.
A) CoCl2
B) CuCl
C) CuClO2
D) CuCl2
E) CoCl
A) CoCl2
B) CuCl
C) CuClO2
D) CuCl2
E) CoCl

Unlock Deck
Unlock for access to all 80 flashcards in this deck.
Unlock Deck
k this deck


