Deck 2: The Mole
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/126
Play
Full screen (f)
Deck 2: The Mole
1
Which of the following contains the largest number of hydrogen atoms?
A) 1 mol of H2O
B) 0.5 mol of NH3
C) 0.20 mol of CH4
D) 0.20 mol of C6H12O6
E) 0.01 mol of H3PO4
A) 1 mol of H2O
B) 0.5 mol of NH3
C) 0.20 mol of CH4
D) 0.20 mol of C6H12O6
E) 0.01 mol of H3PO4
0.20 mol of C6H12O6
2
How many platinum atoms does 1.00 g of pure platinum contain?
A) 195 Pt atoms
B) 3.09 x 1021 Pt atoms
C) 6.17 x 1021 Pt atoms
D) 1.95 x 1023 Pt atoms
E) 6.02 x 1023 Pt atoms
A) 195 Pt atoms
B) 3.09 x 1021 Pt atoms
C) 6.17 x 1021 Pt atoms
D) 1.95 x 1023 Pt atoms
E) 6.02 x 1023 Pt atoms
3.09 x 1021 Pt atoms
3
There are 16 ounces in a lb and 2.20 lbs in a kg. What would be the value of Avogadro's number if this constant was defined as the number of 12C atoms in 12 ounces of 12C?
A) 0.171 x 1023
B) 6.02 x 1023
C) 28.4 x 1023
D) 171 x 1023
E) 2050 x 1023
A) 0.171 x 1023
B) 6.02 x 1023
C) 28.4 x 1023
D) 171 x 1023
E) 2050 x 1023
171 x 1023
4
If the atomic weight of platinum is 195.08 g/mol, what is the mass in grams of a single platinum atom?
A) 1.62 x 10-22 g
B) 3.24 x 10-22 g
C) 5.13 x 10-3 g
D) 195.08 g
E) none of the above
A) 1.62 x 10-22 g
B) 3.24 x 10-22 g
C) 5.13 x 10-3 g
D) 195.08 g
E) none of the above

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
5
What is the molar mass of an element for which a single atom weighs
3.95 x 10-22 g?
A) 6.56 x 10-46
B) 1.31 x 10-45
C) 3.95 x 10-22
D) 238
E) 476
3.95 x 10-22 g?
A) 6.56 x 10-46
B) 1.31 x 10-45
C) 3.95 x 10-22
D) 238
E) 476

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
6
What is the mass in grams of 0.250 moles of magnesium?
A) 0.0103 g
B) 6.08 g
C) 13.8 g
D) 97.2 g
E) none of these
A) 0.0103 g
B) 6.08 g
C) 13.8 g
D) 97.2 g
E) none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following represents the largest number of moles of molecules?
A) 10 g NH3
B) 10 g N2
C) 10 g Cu
D) 10 g Br2
E) all of these contain the same number of moles
A) 10 g NH3
B) 10 g N2
C) 10 g Cu
D) 10 g Br2
E) all of these contain the same number of moles

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
8
How many of oxygen are in 0.500 grams of CO2?
A) 0.500 x 10 - 2
B) 1.00
C) 0.250
D) 1.14 x 10 - 2
E) 2.27 x 10 - 2
A) 0.500 x 10 - 2
B) 1.00
C) 0.250
D) 1.14 x 10 - 2
E) 2.27 x 10 - 2

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
9
Arrange the following in order of increasing mass:
(1) one F atom
(2) 1 x 10 - 20 mol of F
(3) 1 x 10 - 20 g of F
(4) one F2 molecule
A) 1 < 2 < 3 < 4
B) 1 < 4 < 3 < 2
C) 3 < 1 < 2 < 4
D) 2 < 1 < 3 < 4
E) none of these
(1) one F atom
(2) 1 x 10 - 20 mol of F
(3) 1 x 10 - 20 g of F
(4) one F2 molecule
A) 1 < 2 < 3 < 4
B) 1 < 4 < 3 < 2
C) 3 < 1 < 2 < 4
D) 2 < 1 < 3 < 4
E) none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
10
What is the molar mass of barium nitrate, Ba(NO3)2?
A) 261 g/mole
B) 199 g/mole
C) 213 g/mole
D) 167 g/mole
E) none of these
A) 261 g/mole
B) 199 g/mole
C) 213 g/mole
D) 167 g/mole
E) none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
11
What is the mass in grams of 0.125 moles of calcium chloride, CaCI2.
A) 111 g
B) 13.9 g
C) 8.88 x 102 g
D) 1.13 x 10-3 g
E) 41.7 g
A) 111 g
B) 13.9 g
C) 8.88 x 102 g
D) 1.13 x 10-3 g
E) 41.7 g

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
12
How many moles of phosphorus atoms are in 100 grams of P4S10?
A) 4.00
B) 4.00 x 102
C) 0.225
D) 0.487
E) 0.900
A) 4.00
B) 4.00 x 102
C) 0.225
D) 0.487
E) 0.900

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
13
Determine the number of moles in 12.5 g of carbon tetrachloride, CCl4.
A) 8.13 x 10-2 mol
B) 12.3 mol
C) 1.92 x103 mol
D) 7.53 x 1024 mol
E) none of these
A) 8.13 x 10-2 mol
B) 12.3 mol
C) 1.92 x103 mol
D) 7.53 x 1024 mol
E) none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
14
What is the mass in grams of of 0.158 moles of Cu(NO3)2?
A) 2.96 x 101g
B) 8.42 x 10-4 g
C) 1.19 x 103 g
D) 12.3 g
E) none of these
A) 2.96 x 101g
B) 8.42 x 10-4 g
C) 1.19 x 103 g
D) 12.3 g
E) none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
15
Calculate the number of moles in 15.0 grams of NaOH.
A) 40.0 mol
B) 6.00 x 102 mol
C) 2.67 mol
D) 0.375 mol
E) none of these
A) 40.0 mol
B) 6.00 x 102 mol
C) 2.67 mol
D) 0.375 mol
E) none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following contains more atoms of nitrogen, 100 moles of NH3 or 100 moles of N2O5? Explain your reasoning without showing calculations.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
17
How many moles of Na2S are found in 20.0 grams of sodium sulfide, Na2S?
A) 0.256 moles
B) 0.364 moles
C) 2.75 moles
D) 3.90 moles
E) none of these
A) 0.256 moles
B) 0.364 moles
C) 2.75 moles
D) 3.90 moles
E) none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
18
Calculate the number of in 0.125 of barium nitrate, Ba(NO3)2.
A) 2.00 atoms
B) 0.125 atoms
C) 0.250 atoms
D) 1.51 x 1023 atoms
E) 7.53 x 1022 atoms
A) 2.00 atoms
B) 0.125 atoms
C) 0.250 atoms
D) 1.51 x 1023 atoms
E) 7.53 x 1022 atoms

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
19
What is the molecular weight of aspirin, C9H8O4?
A) less than 50 amu
B) between 50 and 80 amu
C) between 80 and 120 amu
D) between 120 and 160 amu
E) more than 160 amu
A) less than 50 amu
B) between 50 and 80 amu
C) between 80 and 120 amu
D) between 120 and 160 amu
E) more than 160 amu

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
20
Cocaine is extracted from the leaves of plants of the species E. coca, whereas chocolate is extracted from the seeds of the species E. cacao. (Never confuse one with the other!) Calculate the molar mass of cocaine, assuming a molecular formula of C17H21NO4.
A) between 0 and 100 g/mol
B) between 100 and 250 g/mol
C) between 250 and 300 g/mol
D) between 300 and 350 g/mol
E) more than 350 g/mol
A) between 0 and 100 g/mol
B) between 100 and 250 g/mol
C) between 250 and 300 g/mol
D) between 300 and 350 g/mol
E) more than 350 g/mol

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
21
What is the mass in amu of one molecule of sucrose (cane sugar) that has the formula: C12H22O11?
A) less than 100 amu
B) between 100 and 150 amu
C) between 150 and 250 amu
D) between 250 and 350 amu
E) more than 350 amu
A) less than 100 amu
B) between 100 and 150 amu
C) between 150 and 250 amu
D) between 250 and 350 amu
E) more than 350 amu

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
22
What is the mass in grams of one molecule of CO2?
A) 4.6 x 10-23 g
B) 7.3 x 10-23 g
C) 28 g
D) 44 g
E) none of the above
A) 4.6 x 10-23 g
B) 7.3 x 10-23 g
C) 28 g
D) 44 g
E) none of the above

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
23
The drug for which more prescriptions - and more bad jokes - were written than any drug released recently has the scientific name Sildenafil citrate and the trade name Viagra™. The structure of Sildenafil is shown below.
 When this structure is translated into a chemical formula we get a molecular formula of C22H32N6O4S. Calculate the molecular weight of this compound.
When this structure is translated into a chemical formula we get a molecular formula of C22H32N6O4S. Calculate the molecular weight of this compound.
A) between 100 and 200 g/mol
B) between 200 and 300 g/mol
C) between 300 and 400 g/mol
D) between 400 and 500 g/mol
E) more than 500 g/mol
 When this structure is translated into a chemical formula we get a molecular formula of C22H32N6O4S. Calculate the molecular weight of this compound.
When this structure is translated into a chemical formula we get a molecular formula of C22H32N6O4S. Calculate the molecular weight of this compound.A) between 100 and 200 g/mol
B) between 200 and 300 g/mol
C) between 300 and 400 g/mol
D) between 400 and 500 g/mol
E) more than 500 g/mol

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
24
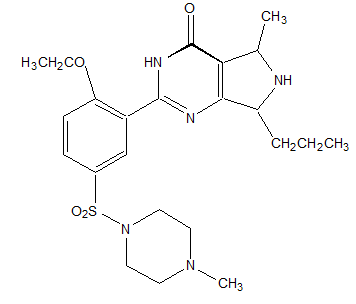
Let's consider another "miracle drug" with the scientific name orlistat, which is marketed by Roche Pharmaceuticals as Xenical. The formal chemical name for Xenical is (s)-2-formylamino-4-methyl-pentanoic acid (s)-1-[[(2S, 3S)-3-hexyl-4-oxo-2-oxetanyl] methyl]-dodecyl ester. Its structure is shown below. ![<strong>Let's consider another miracle drug with the scientific name orlistat, which is marketed by Roche Pharmaceuticals as Xenical<sup></sup>. The formal chemical name for Xenical<sup></sup> is (s)-2-formylamino-4-methyl-pentanoic acid (s)-1-[[(2S, 3S)-3-hexyl-4-oxo-2-oxetanyl] methyl]-dodecyl ester. Its structure is shown below. The molecular formula for this wonder diet drug that is supposed to block the uptake of fat is C<sub>29</sub>H<sub>35</sub>NO<sub>5</sub>. Calculate the molecular weight of this compound.</strong> A) between 100 and 200 g/mol B) between 200 and 300 g/mol C) between 300 and 400 g/mol D) between 400 and 500 g/mol E) more than 500 g/mol](https://storage.examlex.com/TB9692/11efbdf5_d9cc_cf5c_b4ad_fbf62a49a6c4_TB9692_00.jpg) The molecular formula for this wonder diet drug that is supposed to block the uptake of fat is C29H35NO5. Calculate the molecular weight of this compound.
The molecular formula for this wonder diet drug that is supposed to block the uptake of fat is C29H35NO5. Calculate the molecular weight of this compound.
A) between 100 and 200 g/mol
B) between 200 and 300 g/mol
C) between 300 and 400 g/mol
D) between 400 and 500 g/mol
E) more than 500 g/mol
![<strong>Let's consider another miracle drug with the scientific name orlistat, which is marketed by Roche Pharmaceuticals as Xenical<sup></sup>. The formal chemical name for Xenical<sup></sup> is (s)-2-formylamino-4-methyl-pentanoic acid (s)-1-[[(2S, 3S)-3-hexyl-4-oxo-2-oxetanyl] methyl]-dodecyl ester. Its structure is shown below. The molecular formula for this wonder diet drug that is supposed to block the uptake of fat is C<sub>29</sub>H<sub>35</sub>NO<sub>5</sub>. Calculate the molecular weight of this compound.</strong> A) between 100 and 200 g/mol B) between 200 and 300 g/mol C) between 300 and 400 g/mol D) between 400 and 500 g/mol E) more than 500 g/mol](https://storage.examlex.com/TB9692/11efbdf5_d9cc_cf5c_b4ad_fbf62a49a6c4_TB9692_00.jpg) The molecular formula for this wonder diet drug that is supposed to block the uptake of fat is C29H35NO5. Calculate the molecular weight of this compound.
The molecular formula for this wonder diet drug that is supposed to block the uptake of fat is C29H35NO5. Calculate the molecular weight of this compound.A) between 100 and 200 g/mol
B) between 200 and 300 g/mol
C) between 300 and 400 g/mol
D) between 400 and 500 g/mol
E) more than 500 g/mol

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
25
If you eat too much fat while taking Xenical, you're going to be very, very "uncomfortable." You can avoid this problem, of course, by taking the other new diet drug, Sibutramine hydrochloride monohydrate, which is marketed by a subsidiary of BASF as Meridia. Meridia is a neurotransmitter that inhibits the uptake of other neurotransmitters, such as dopamine, norepinephrine, and serotonin. It therefore tends to depress one's appetite and is being studied as a possible treatment for depression. Elemental analysis suggests that this compound is 72.96% C, 9.36% H, 12.67% Cl, and 5.01% N by weight. What is the total number of C, H, N and Cl atoms in the molecular formula of this compound if the molecular weight is 279.85 g/mol?
A) 10 or less
B) between 11 and 20
C) between 21 and 30
D) between 31 and 40
E) between 41 and 50
A) 10 or less
B) between 11 and 20
C) between 21 and 30
D) between 31 and 40
E) between 41 and 50

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
26
ADM (Archer Daniels Midland) the self-proclaimed "Supermarket to the World" has been in trouble for allegedly fixing the price of lysine, an amino acid that is often deficient in vegetarian diets. Lysine is 19.16% N by weight and each molecule contains two nitrogen atoms. Calculate the molecular weight of lysine.
A) less than 100 g/mol
B) between 100 and 150 g/mol
C) between 150 and 250 g/mol
D) between 250 and 350 g/mol
E) more than 350 g/mol
A) less than 100 g/mol
B) between 100 and 150 g/mol
C) between 150 and 250 g/mol
D) between 250 and 350 g/mol
E) more than 350 g/mol

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
27
Calculate the number of carbon atoms in a mole of sucrose, C12H22O11.
A) 12 atoms
B) 6.02 x 1023 atoms
C) 6.62 x 1024 atoms
D) 7.22 x 1024 atoms
E) 1.3 x 1025 atoms
A) 12 atoms
B) 6.02 x 1023 atoms
C) 6.62 x 1024 atoms
D) 7.22 x 1024 atoms
E) 1.3 x 1025 atoms

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
28
Calculate the number of chlorine atoms in 10.4 grams of chloroform, CHCl3.
A) 5.25 x 1022 atoms
B) 1.05 x 1023 atoms
C) 1.57 x 1023 atoms
D) 2.10 x 1023 atoms
E) 2.07 x 1025 atoms
A) 5.25 x 1022 atoms
B) 1.05 x 1023 atoms
C) 1.57 x 1023 atoms
D) 2.10 x 1023 atoms
E) 2.07 x 1025 atoms

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
29
Sodium tripolyphosphate, Na5P3O10, is added to detergents to increase their cleaning power. Calculate the number of phosphorus atoms in 0.325 moles of this compound.
A) 6.52 x 1022 atoms
B) 1.96 x 1023 atoms
C) 5.87 x 1023 atoms
D) 6.02 x 1023 atoms
E) none of the above
A) 6.52 x 1022 atoms
B) 1.96 x 1023 atoms
C) 5.87 x 1023 atoms
D) 6.02 x 1023 atoms
E) none of the above

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
30
Acetic acid, C2H4O2, is the substance that makes vinegar taste sour. What is the % mass of carbon in acetic acid?
A) 20%
B) 25%
C) 35%
D) 40%
E) none of these
A) 20%
B) 25%
C) 35%
D) 40%
E) none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
31
Calculate the % mass of sodium in sodium sulfate, Na2SO4.
A) 16%
B) 23%
C) 32%
D) 54%
E) 67%
A) 16%
B) 23%
C) 32%
D) 54%
E) 67%

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
32
A hydrate is heated to determine the % water by mass in the hydrate. During heating of the hydrate a small chip is broken off of the lid of the crucible and falls to the lab bench without being noticed. What effect will this have on the calculated value of the % water by mass?
A) It will be high.
B) It will be low.
C) It will not affect the results.
D) It could be either high or low.
A) It will be high.
B) It will be low.
C) It will not affect the results.
D) It could be either high or low.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following nitrogen-containing compounds would supply the most nitrogen per gram of fertilizer?
A) iron azide, Fe(N3)2, MW = 140 g/mol
B) sodium azide, NaN3, MW = 65 g/mol
C) potassium nitrite, KNO2, MW = 85.1 g/mol
D) potassium azide, KN3, MW = 81.1 g/mol
E) There is not enough information to answer this problem.
A) iron azide, Fe(N3)2, MW = 140 g/mol
B) sodium azide, NaN3, MW = 65 g/mol
C) potassium nitrite, KNO2, MW = 85.1 g/mol
D) potassium azide, KN3, MW = 81.1 g/mol
E) There is not enough information to answer this problem.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
34
Apatite is a mineral that is found in tooth enamel. When fluoride toothpastes are used, this mineral is converted to fluoroapatite, which is much harder and therefore more resistant to decay. What is the percent by weight of fluorine in fluoroapatite, Ca5(PO4)3F?
A) between 0 and 0.1%
B) between 0.1% and 1%
C) between 1% and 3%
D) between 3% and 5%
E) more than 5%
A) between 0 and 0.1%
B) between 0.1% and 1%
C) between 1% and 3%
D) between 3% and 5%
E) more than 5%

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
35
In March of 2007 a number of pets were sickened or killed by pet food contaminated with the chemical melamine which is used in the manufacture of certain plastics. It is thought that the melamine was added to make a pet food additive appear to have more protein than it really did. Protein content is determined by measuring nitrogen content of food since protein is the main source of nitrogen in food. What is the % by mass of nitrogen in melamine which has the formula C3H6N6?
A) 66.6%
B) 61.2%
C) 11.1%
D) 82.3%
E) Insufficient information is given to find the %N by mass.
A) 66.6%
B) 61.2%
C) 11.1%
D) 82.3%
E) Insufficient information is given to find the %N by mass.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
36
In March of 2007 a number of pets were sickened or killed by pet food contaminated with the chemical melamine (C3H6N6) which is used in the manufacture of certain plastics. It is thought that the melamine was added to make a pet food additive appear to have more protein than it really did. Protein content is determined by measuring the %N by mass in food because protein is the main source of nitrogen in food. Proteins are composed of amino acids so the %N by mass of a protein reflects the %N of the individual amino acids. Alanine is a typical amino acid and has the formula C3H7NO2. Calculate the %N by mass in melamine and alanine. Why would adding a small amount of melamine make something appear to have a high protein content?

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
37
Determine the empirical formula of a compound containing 59.0% Na and 41.0% O by mass.
A) NaO
B) Na2O
C) NaO2
D) Na2O3
E) none of these
A) NaO
B) Na2O
C) NaO2
D) Na2O3
E) none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
38
Cobalt oxide used in glazing pottery contains 71.06% cobalt and 28.94% oxygen by mass. What is the empirical formula of this cobalt oxide?
A) CoO
B) Co2O
C) CoO3
D) Co2O3
E) Co3O4
A) CoO
B) Co2O
C) CoO3
D) Co2O3
E) Co3O4

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
39
Calculate the molecular weight of the oxygen-carrier protein hemoglobin if
This protein is 0.335% Fe by weight and each protein molecule contains four
Iron atoms.
A) less than 1000 grams per mole
B) between 1000 and 10,000 grams per mole
C) between 10,000 and 50,000 grams per mole
D) between 50,000 and 100,000 grams per mole
E) more than 100,000 grams per mole
This protein is 0.335% Fe by weight and each protein molecule contains four
Iron atoms.
A) less than 1000 grams per mole
B) between 1000 and 10,000 grams per mole
C) between 10,000 and 50,000 grams per mole
D) between 50,000 and 100,000 grams per mole
E) more than 100,000 grams per mole

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
40
Calculate the number of carbon atoms in the empirical formula of nicotine if this compound is 74.0% C, 8.7% H, and 17.3% N by weight.
A) 3 atoms
B) 4 atoms
C) 5 atoms
D) 7 atoms
E) 10 atoms
A) 3 atoms
B) 4 atoms
C) 5 atoms
D) 7 atoms
E) 10 atoms

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
41
Glucose (blood sugar), acetic acid, and formaldehyde are all 40.0% C, 6.76% H, and 53.3% O by weight. Calculate the total number of carbon, hydrogen, and oxygen atoms in the empirical formula for these compounds.
A) 3
B) 4
C) 5
D) 6
E) 9
A) 3
B) 4
C) 5
D) 6
E) 9

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
42
A compound that is 43.64% P and 56.36% O by weight has a molecular
Weight of 283.88 g/mol. What is the molecular formula of this compound?
A) PO4
B) P2O3
C) P2O5
D) P4O10
E) none of the above
Weight of 283.88 g/mol. What is the molecular formula of this compound?
A) PO4
B) P2O3
C) P2O5
D) P4O10
E) none of the above

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
43
A mixed oxide of potassium and vanadium is 28.3% by weight potassium and 37.0% by weight vanadium. What is the empirical formula?
A) KV
B) KVO
C) K2V3O
D) K3V3O
E) KVO3
A) KV
B) KVO
C) K2V3O
D) K3V3O
E) KVO3

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
44
The chief ore of manganese is an oxide known as pyrolusite, which is 36.8% O and 63.2% Mn by weight. Which of the following is the correct empirical formula for pyrolusite?
A) MnO
B) MnO2
C) Mn2O3
D) MnO3
E) Mn2O7
A) MnO
B) MnO2
C) Mn2O3
D) MnO3
E) Mn2O7

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
45
Nitrogen combines with oxygen to form a variety of compounds. One of these compounds is called nitrous oxide or "laughing gas." What is the formula of nitrous oxide if this compound is 63.65% N and 36.35% O by weight?
A) N2O
B) NO
C) NO2
D) N2O3
E) N2O5
A) N2O
B) NO
C) NO2
D) N2O3
E) N2O5

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
46
What is the atomic weight of a metal that forms an oxide, M2O3, which is 17.29% oxygen by weight?
A) 38.3 g/mol
B) 57.4 g/mol
C) 76.6 g/mol
D) 115 g/mol
E) 229 g/mol
A) 38.3 g/mol
B) 57.4 g/mol
C) 76.6 g/mol
D) 115 g/mol
E) 229 g/mol

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
47
Cisplatin is a commercial antitumor drug that contains 65.0% Pt, 23.7% Cl, 9.3% N and 2.0% H by weight. What is the ratio of H to Pt atoms in the empirical formula of this compound?
A) 1:1
B) 2:1
C) 3:1
D) 4:1
E) 6:1
A) 1:1
B) 2:1
C) 3:1
D) 4:1
E) 6:1

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
48
What is the atomic weight in amu of the element that forms a fluoride, M2F4, which is 73.1% fluorine by weight?
A) 14.0 amu
B) 16.0 amu
C) 28.0 amu
D) 32.0 amu
E) none of these
A) 14.0 amu
B) 16.0 amu
C) 28.0 amu
D) 32.0 amu
E) none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
49
What is the empirical formula of a compound that contains C, H, and O if the compound is 40.00% C and 6.71% H by weight?
A) CHO
B) CH2O
C) CH3O
D) CH4O
E) none of the above
A) CHO
B) CH2O
C) CH3O
D) CH4O
E) none of the above

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
50
Calculate the empirical formula of naphthalene if this compound is 93.71% carbon and 6.29% hydrogen by weight.
A) CH
B) CH2
C) C3H2
D) C4H5
E) C5H4
A) CH
B) CH2
C) C3H2
D) C4H5
E) C5H4

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
51
The molecular weight of naphthalene is 128.17 grams per mole. Use the results of the previous question to calculate the total number of atoms in a naphthalene molecule.
A) 9 atoms
B) 12 atoms
C) 18 atoms
D) 27 atoms
E) 38 atoms
A) 9 atoms
B) 12 atoms
C) 18 atoms
D) 27 atoms
E) 38 atoms

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
52
The term "carbohydrate" once meant a compound with the empirical formula CH2O. What is the molecular formula of glucose (blood sugar) if the molecular weight of this carbohydrate is 180 g/mol?
A) CH2O
B) C4H8O4
C) C6H12O6
D) C12H22O11
E) none of the above
A) CH2O
B) C4H8O4
C) C6H12O6
D) C12H22O11
E) none of the above

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
53
A 1.854 g sample of D-ribose contains 0.01236 moles of this sugar which has an empirical formula of CH2O. What is the ratio of the molecular formula to the empirical formula of this compound?
A) 1
B) 2
C) 3
D) 5
E) none of these
A) 1
B) 2
C) 3
D) 5
E) none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
54
The ribonucleic acids (RNA) that are so important in the biosynthesis of proteins all contain the sugar D-ribose, which contains three elements: C, H, and O. Elemental analysis suggests that D-ribose is 40.00% C and 6.71% H by weight. What is the empirical formula of this compound?
A) CHO
B) CH2O
C) CH3O
D) CH3O2
E) none of these
A) CHO
B) CH2O
C) CH3O
D) CH3O2
E) none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
55
Halothane is an anesthetic that is 12.17% C, 0.51% H, 40.48% Br, 17.96% Cl and 28.87% F by weight. Calculate the molecular formula of this compound if each molecule contains one hydrogen atom.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
56
A compound frequently used as a high-temperature lubricant has the formula MS2, where M represents some metallic element. If this compound is 40.06% sulfur by mass, what is the identity of the metal M?
A) K
B) Fe
C) Cu
D) Mo
E) Pb
A) K
B) Fe
C) Cu
D) Mo
E) Pb

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
57
In small quantities, the nicotine in tobacco is addictive. In large quantities, it is a deadly poison. Calculate the molecular formula of nicotine, CxHyNz, if the molecular weight is 162.2 g/mol and 0.438 grams of this compound burn to form 1.188 grams of CO2 and 0.341 grams of water.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
58
In 1763, the Reverend Edmund Stone suggested chewing the bark of the English willow (Salix abla) to treat a fever because it grows in moist regions where one was likely to catch a fever, and "... many natural maladies carry their cures along with them, or their remedies lie not far from their causes." It took 50 years to isolate the active ingredient in the bark, which was named salicylic acid. By the end of the 19th century, salicylic acid was used to treat rheumatic fever, gout and arthritis. Many patients complained of chronic stomach irritation, however. Because his father was one of these patients, Felix Hoffman searched for a less acidic derivative of salicylic acid. In 1898, Hoffman reported that the acetyl ester of salicylic acid was both more effective and easier to tolerate than the parent compound. He named the compound aspirin, taking the prefix a- from the name of the acetyl group and spirin from the German name of the parent compound spirsure. Aspirin is 60.00% C, 4.48% H, and 35.5% O by weight. What is the total number of C, H and O atoms in the empirical formula of this compound?
A) less than 15 atoms
B) between 15 and 20 atoms
C) between 20 and 25 atoms
D) between 25 and 35 atoms
E) more than 35 atoms
A) less than 15 atoms
B) between 15 and 20 atoms
C) between 20 and 25 atoms
D) between 25 and 35 atoms
E) more than 35 atoms

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
59
One of the concerns during the war against Iraq several years ago was fear of chemical warfare agents, such as the nerve agent VX. VX is the most dangerous material ever synthesized; a single drop of VX on the skin will kill an individual within hours. More than 2 million pounds of VX were made in Southern Indiana by 1968. They are still there, stored in 1600 canisters, packed three high. The canisters were welded together several years ago because of fear of exposure to VX if a tornado struck the storage facility. Elemental analysis of VX gives the following result: 49.41% C, 9.80% H, 5.24% N, 11.97% O, 11.58% P, and 11.99% S. How many carbon atoms does the empirical formula for this compound contain?
(Hint: Carry out the calculation to at least three significant figures.)
A) 3 or less atoms
B) 4 to 7 atoms
C) 8 to 11 atoms
D) 12 to 15 atoms
E) more than 15 atoms
(Hint: Carry out the calculation to at least three significant figures.)
A) 3 or less atoms
B) 4 to 7 atoms
C) 8 to 11 atoms
D) 12 to 15 atoms
E) more than 15 atoms

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
60
Assume that the empirical formula of VX (described in the previous question) is the same as the molecular formula. What is the molecular weight of VX?
A) less than 150 grams/mole
B) between 150 and 200 grams/mole
C) between 200 and 250 grams/mole
D) between 250 and 300 grams/mole
E) more than 300 grams/mole
A) less than 150 grams/mole
B) between 150 and 200 grams/mole
C) between 200 and 250 grams/mole
D) between 250 and 300 grams/mole
E) more than 300 grams/mole

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
61
On March 20, 1995, terrorists released an organophosphate nerve gas known as "Sarin" at several points in the Tokyo subway system, killing 11 and injuring more than 5,500 people. Sarin was concealed in lunch boxes and soft-drink containers and placed on subway train floors. It was released as terrorists punctured the containers with umbrellas before leaving the trains. Elemental analysis of Sarin gives the following result: 34.29% C, 7.19% H, 22.84% O, 13.56% F, 22.11% P. How many carbon atoms does the empirical formula of this compound contain?
A) 2 or less atoms
B) 3 to 6 atoms
C) 7 to 10 atoms
D) 11 to 15 atoms
E) more than 15 atoms
A) 2 or less atoms
B) 3 to 6 atoms
C) 7 to 10 atoms
D) 11 to 15 atoms
E) more than 15 atoms

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
62
A sample of copper(II) sulfate, CuSO4, that weighs 2.47 grams picks up
Water from the atmosphere to form a hydrate with the formula CuSO4 • x H2O. If the sample weighs 3.86 grams after it picks up water, what is the value of x?
A) 2
B) 2.5
C) 3
D) 4
E) 5
Water from the atmosphere to form a hydrate with the formula CuSO4 • x H2O. If the sample weighs 3.86 grams after it picks up water, what is the value of x?
A) 2
B) 2.5
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
63
The thermal decomposition of carbonates leads to the loss of CO2. The decomposition of an unknown carbonate leads to a 35.1% weight loss. The unknown was which of the following compounds.
A) Li2CO3
B) MgCO3
C) CaCO3
D) ZnCO3
E) BaCO3
A) Li2CO3
B) MgCO3
C) CaCO3
D) ZnCO3
E) BaCO3

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
64
In March of 2007 a number of pets were sickened or killed by pet food contaminated with the chemical melamine which is used in the manufacture of certain plastics. It is thought that the melamine was added to make a pet food additive appear to have more protein than it really did. Melamine is 28.57% C, 4.80% H, and 66.63% N by mass. What is melamine's empirical formula?
A) C9H5N7
B) CHN2
C) C2HN7
D) CH2N2
E) CHN
A) C9H5N7
B) CHN2
C) C2HN7
D) CH2N2
E) CHN

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
65
In March of 2007 a number of pets were sickened or killed by pet food contaminated with the chemical melamine which is used in the manufacture of certain plastics. It is thought that the melamine was added to make a pet food additive appear to have more protein than it really did. Melamine has the empirical formula CH2N2. If the molecular weight of melamine is 126.15 g/mol, what is its molecular formula?
A) CH2N2
B) CH3N3
C) C3H6N6
D) C2H4N4
E) C4H8N8
A) CH2N2
B) CH3N3
C) C3H6N6
D) C2H4N4
E) C4H8N8

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
66
If 0.50 moles of PbS are combined with 0.50 moles of O2, how many moles of PbO can be produced by the following reaction?
2 PbS(s) + 3 O2(g) 2 PbO(s) + 2 SO2(g)
A) 0.33
B) 0.50
C) 0.75
D) 2.0
E) none of these
2 PbS(s) + 3 O2(g) 2 PbO(s) + 2 SO2(g)
A) 0.33
B) 0.50
C) 0.75
D) 2.0
E) none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
67
What would happen to the potential yield of sulfur dioxide in the previous question if the amount of oxygen was doubled?
A) It would decrease by a factor of 2.
B) It would decrease by a factor of 1.5.
C) It would remain constant.
D) It would increase by a factor of 1.5.
E) It would increase by a factor of 2.
A) It would decrease by a factor of 2.
B) It would decrease by a factor of 1.5.
C) It would remain constant.
D) It would increase by a factor of 1.5.
E) It would increase by a factor of 2.

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
68
Acetylene gas, C2H2, burns in the presence of oxygen by the following reaction:
2 C2H2(g) + 5 O2(g) 2 H2O(l) + 4 CO2(g)
If 4.5 moles of acetylene gas are to be burned, how many moles of oxygen will be required for the complete reaction of the acetylene?
A) 4.5 moles
B) 23 moles
C) 8.1 moles
D) 5.0 moles
E) none of these
2 C2H2(g) + 5 O2(g) 2 H2O(l) + 4 CO2(g)
If 4.5 moles of acetylene gas are to be burned, how many moles of oxygen will be required for the complete reaction of the acetylene?
A) 4.5 moles
B) 23 moles
C) 8.1 moles
D) 5.0 moles
E) none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
69
For the reaction: 4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(l), how many moles of ammonia, NH3, are required to react completely with 0.250 moles of oxygen?
A) 0.400
B) 0.500
C) 0.200
D) 0.313
E) 0.178
A) 0.400
B) 0.500
C) 0.200
D) 0.313
E) 0.178

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
70
The molecular formula of isooctane is C8H18. It burns in the presence of oxygen to form a mixture of CO2 and H2O. What is the ratio of moles of water to moles of carbon dioxide produced in this reaction?
A) 1:1
B) 2:1
C) 9:4
D) 9:8
E) none of the above
A) 1:1
B) 2:1
C) 9:4
D) 9:8
E) none of the above

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
71
A laboratory method for preparing oxygen (O2) involves the decomposition of KClO3.
KClO3(s) 2 KCI(s) + 3 O2(g)
How many moles of oxygen are produced when 0.135 moles of KCl are produced by this reaction?
A) 0.135 mol
B) 0.090 mol
C) 0.203 mol
D) 0.405 mol
E) none of these
KClO3(s) 2 KCI(s) + 3 O2(g)
How many moles of oxygen are produced when 0.135 moles of KCl are produced by this reaction?
A) 0.135 mol
B) 0.090 mol
C) 0.203 mol
D) 0.405 mol
E) none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
72
What mass of O2, in grams, is produced in the decomposition of 100 g of KClO3 by the following reaction?
2 KClO3(s) 2 KCl(s) + 3 O2(g)
A) 0.816 grams
B) 1.22 grams
C) 39.2 grams
D) 1.50 x 102 grams
E) 26.1 grams
2 KClO3(s) 2 KCl(s) + 3 O2(g)
A) 0.816 grams
B) 1.22 grams
C) 39.2 grams
D) 1.50 x 102 grams
E) 26.1 grams

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
73
Iron ore is impure Fe2O3. When Fe2O3 is heated with an excess of carbon (coke), iron metal is produced. How many moles of Fe can be produced from 250 g of Fe2O3 by the following reaction?
Fe2O3 (s) + 3 C(s) 2 Fe(l) + 3 CO(g)
A) 5.00 x 102 mol
B) 125 mol
C) 1.57 mol
D) 3.13 mol
E) 0.785 mol
Fe2O3 (s) + 3 C(s) 2 Fe(l) + 3 CO(g)
A) 5.00 x 102 mol
B) 125 mol
C) 1.57 mol
D) 3.13 mol
E) 0.785 mol

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
74
Calculate the number of moles of iron, Fe, produced when 0.500 moles of carbon monoxide, CO, react with an excess of iron oxide.
Fe2O3(s) + 3 CO(g) 2 Fe(s) + 3 CO2(g)
A) 2.00 moles
B) 1.00 moles
C) 0.750 moles
D) 0.166 moles
E) none of these
Fe2O3(s) + 3 CO(g) 2 Fe(s) + 3 CO2(g)
A) 2.00 moles
B) 1.00 moles
C) 0.750 moles
D) 0.166 moles
E) none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
75
Hydrogen chloride, HCI, can be made by the reaction of phosphorus trichloride, PCl3, with water and then boiling the HCI gas out of the solution. Calculate the mass of HCI gas that can be prepared from 15.0 g of PCl3 and an excess of water.
PCl3(g) + 3 H2O(l) 3 HCI(aq) + H3PO3(aq)
A) 0.328 g
B) 3.97 g
C) 11.9 g
D) 35.7 g
E) 3.00 g
PCl3(g) + 3 H2O(l) 3 HCI(aq) + H3PO3(aq)
A) 0.328 g
B) 3.97 g
C) 11.9 g
D) 35.7 g
E) 3.00 g

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
76
Ethyl alcohol, C2H5OH, is also known as drinking alcohol. It can be produced by the fermentation of glucose by the following reaction.
C6H12O6(aq) 2 C2H5OH(aq) + 2 CO2(g)
How many of ethanol can be produced from of glucose?
A) 100 moles
B) 200 moles
C) 0.556 moles
D) 1.11 moles
E) none of these
C6H12O6(aq) 2 C2H5OH(aq) + 2 CO2(g)
How many of ethanol can be produced from of glucose?
A) 100 moles
B) 200 moles
C) 0.556 moles
D) 1.11 moles
E) none of these

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
77
Nitrogen reacts with red-hot magnesium to form magnesium nitride,
3 Mg(s) + N2(g) Mg3N2(s)
Which reacts with water to form magnesium hydroxide and ammonia,
Mg3N2(s) + 6 H2O(l) 3 Mg(OH)2(aq) + 2 NH3(aq)
How many grams of magnesium would you have to start with to prepare 15.0 grams of ammonia?
A) 13.3 g
B) 15.0 g
C) 20.0 g
D) 32.2 g
E) none of the above
3 Mg(s) + N2(g) Mg3N2(s)
Which reacts with water to form magnesium hydroxide and ammonia,
Mg3N2(s) + 6 H2O(l) 3 Mg(OH)2(aq) + 2 NH3(aq)
How many grams of magnesium would you have to start with to prepare 15.0 grams of ammonia?
A) 13.3 g
B) 15.0 g
C) 20.0 g
D) 32.2 g
E) none of the above

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
78
The chemical formula of ethanol is C2H6O. It burns in the presence of excess oxygen to form CO2 and H2O. How many grams of H2O are produced from the combustion of 25.0 g of ethanol?
A) 9.78 g
B) 18.0 g
C) 25.0 g
D) 29.3 g
E) 54.1 g
A) 9.78 g
B) 18.0 g
C) 25.0 g
D) 29.3 g
E) 54.1 g

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
79
Sucrose, or "cane sugar," has the formula C12H22O11. What weight of carbon dioxide can be produced from 10.0 grams of sucrose and 10.0 grams of oxygen?
C12H22O11(s) + 12 O2(g) 12 CO2(g) + 11 H2O(g)
A) less than 5 grams
B) between 5 and 10 grams
C) between 10 and 15 grams
D) between 15 and 20 grams
E) more than 20 grams
C12H22O11(s) + 12 O2(g) 12 CO2(g) + 11 H2O(g)
A) less than 5 grams
B) between 5 and 10 grams
C) between 10 and 15 grams
D) between 15 and 20 grams
E) more than 20 grams

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck
80
PF3 reacts with XeF4 to give PF5. In theory, how many moles of PF5 can be produced from 100.0 g of PF3 and 50.0 g of XeF4?
2 PF3(g) + XeF4(s) 2 PF5(g) + Xe(g)
A) 0.121 mol
B) 0.241 mol
C) 0.482 mol
D) 1.14 mol
E) 2.28 mol
2 PF3(g) + XeF4(s) 2 PF5(g) + Xe(g)
A) 0.121 mol
B) 0.241 mol
C) 0.482 mol
D) 1.14 mol
E) 2.28 mol

Unlock Deck
Unlock for access to all 126 flashcards in this deck.
Unlock Deck
k this deck


