Deck 29: The Nucleus
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/63
Play
Full screen (f)
Deck 29: The Nucleus
1
The normal U.S. average annual radiation dose per person is about how many REM?
0.20
2
Discuss what observations convinced Rutherford that the nucleus was very small and dense.
Alpha scattering from heavy nuclei produced much more backscattering than would be expected from a diffuse nuclear cloud. Only a dense compact nucleus of positive charge could account for the scattering at large angles.
3
Why do the high-proton-number elements tend to get more unstable?
The repulsive electrostatic force between protons increases progressively as atomic number increases, but the nuclear attractive force is short range and the average distance between particles increases as the nucleus size increases.
4
Name five different types of radiation detectors.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
5
J. J. Thomson
A) observed back-scattering from nuclei.
B) proposed the tiny central nucleus idea.
C) proposed the plum-pudding model.
D) discovered radium and polonium.
E) invented the particle detector tube.
A) observed back-scattering from nuclei.
B) proposed the tiny central nucleus idea.
C) proposed the plum-pudding model.
D) discovered radium and polonium.
E) invented the particle detector tube.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
6
Ernest Rutherford
A) observed back-scattering from nuclei.
B) proposed the tiny central nucleus idea.
C) proposed the plum-pudding model.
D) invented the particle detector tube.
E) discovered radium and polonium.
A) observed back-scattering from nuclei.
B) proposed the tiny central nucleus idea.
C) proposed the plum-pudding model.
D) invented the particle detector tube.
E) discovered radium and polonium.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
7
Compared to the electrostatic force, the nuclear force between adjacent protons in a nucleus is
A) about the same size.
B) only slightly weaker.
C) only slightly larger.
D) much weaker.
E) much stronger.
A) about the same size.
B) only slightly weaker.
C) only slightly larger.
D) much weaker.
E) much stronger.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
8
The number of protons in a neutral atom is
A) equal to the number of neutrons.
B) equal to the number of electrons.
C) called the mass number.
D) the same for all elements.
E) zero.
A) equal to the number of neutrons.
B) equal to the number of electrons.
C) called the mass number.
D) the same for all elements.
E) zero.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
9
An atom's mass number is determined by the number of
A) protons in its nucleus.
B) nucleons in its nucleus.
C) electron masses in its nucleus.
D) neutrons in its nucleus.
E) alpha particles in its nucleus.
A) protons in its nucleus.
B) nucleons in its nucleus.
C) electron masses in its nucleus.
D) neutrons in its nucleus.
E) alpha particles in its nucleus.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
10
Isotopes of an element have nuclei with
A) a different number of protons, and the same numbers of neutrons.
B) the same number of protons, but different numbers of electrons.
C) a different number of protons, and a different number of neutrons.
D) the same number of protons, but different numbers of neutrons.
E) the same number of protons, and the same number of neutrons.
A) a different number of protons, and the same numbers of neutrons.
B) the same number of protons, but different numbers of electrons.
C) a different number of protons, and a different number of neutrons.
D) the same number of protons, but different numbers of neutrons.
E) the same number of protons, and the same number of neutrons.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
11
All isotopes are unstable which have proton number greater than
A) 83.
B) 12.
C) 73.
D) 78.
E) 26.
A) 83.
B) 12.
C) 73.
D) 78.
E) 26.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
12
When an alpha particle is emitted from an unstable nucleus, the atomic mass number of the nucleus
A) increases by 2.
B) decreases by 4.
C) stays unchanged.
D) decreases by 2.
E) increases by 4.
A) increases by 2.
B) decreases by 4.
C) stays unchanged.
D) decreases by 2.
E) increases by 4.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
13
When a neutron is emitted from an unstable nucleus, the atomic mass number of the nucleus
A) does not change.
B) decreases by 2.
C) increases by 2.
D) increases by 1.
E) decreases by 1.
A) does not change.
B) decreases by 2.
C) increases by 2.
D) increases by 1.
E) decreases by 1.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
14
When a + particle is emitted from an unstable nucleus, the atomic number of the nucleus
A) increases by 2.
B) does not change.
C) decreases by 2.
D) increases by 1.
E) decreases by 1.
A) increases by 2.
B) does not change.
C) decreases by 2.
D) increases by 1.
E) decreases by 1.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
15
Marie & Pierre Curie
A) discovered radioactivity.
B) proposed the plum-pudding model.
C) observed back-scattering from nuclei.
D) discovered radium and polonium.
E) proposed the tiny central nucleus idea.
A) discovered radioactivity.
B) proposed the plum-pudding model.
C) observed back-scattering from nuclei.
D) discovered radium and polonium.
E) proposed the tiny central nucleus idea.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
16
Henri Becquerel
A) proposed the tiny central nucleus idea.
B) discovered radioactivity.
C) invented the particle detector tube.
D) discovered radium and polonium.
E) observed back-scattering from nuclei.
A) proposed the tiny central nucleus idea.
B) discovered radioactivity.
C) invented the particle detector tube.
D) discovered radium and polonium.
E) observed back-scattering from nuclei.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
17
Which isotopes are radioactive?
A) none
B) some
C) odd numbered
D) even numbered
E) all
A) none
B) some
C) odd numbered
D) even numbered
E) all

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
18
When an alpha particle is emitted from an unstable nucleus, the atomic number of the nucleus
A) increases by 2.
B) decreases by 4.
C) decreases by 2.
D) increases by 4.
E) increases by 6.
A) increases by 2.
B) decreases by 4.
C) decreases by 2.
D) increases by 4.
E) increases by 6.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
19
When a nucleus undergoes beta-minus decay, it changes into
A) a higher atomic number.
B) a higher neutron number.
C) a higher mass number.
D) another isotope of itself.
E) a lower proton number.
A) a higher atomic number.
B) a higher neutron number.
C) a higher mass number.
D) another isotope of itself.
E) a lower proton number.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
20
In radioactive dating, carbon-14 is often used. This nucleus emits a single beta particle when it decays. When this happens, the resulting nucleus is
A) carbon-15.
B) carbon-13.
C) nitrogen-14.
D) still carbon-14.
E) boron-14.
A) carbon-15.
B) carbon-13.
C) nitrogen-14.
D) still carbon-14.
E) boron-14.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
21
Cosmic rays react with what atmospheric atoms to produce carbon-14?
A) oxygen
B) carbon-12
C) helium
D) nitrogen
E) hydrogen
A) oxygen
B) carbon-12
C) helium
D) nitrogen
E) hydrogen

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
22
Alpha decay reduces the atomic and mass numbers respectively by how much?
A) 2, 4
B) 4, 2
C) 4, 4
D) 2, 2
A) 2, 4
B) 4, 2
C) 4, 4
D) 2, 2

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
23
A becquerel (Bq) is
A) 3.70 × 1010 decays/s in radium.
B) the SI unit of radioactivity (= 1 decay/s).
C) a dosage of 2.58 × 10-4 C/kg.
D) 10-2 J/kg.
E) the SI unit of absorbed dose = 1 J/kg = 100 rad.
A) 3.70 × 1010 decays/s in radium.
B) the SI unit of radioactivity (= 1 decay/s).
C) a dosage of 2.58 × 10-4 C/kg.
D) 10-2 J/kg.
E) the SI unit of absorbed dose = 1 J/kg = 100 rad.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
24
Carbon-14 dating can reliably date ancient archeological samples up to approximately how many years?
A) 5 thousand
B) 100 million
C) 10 billion
D) 50 thousand
E) 5 million
A) 5 thousand
B) 100 million
C) 10 billion
D) 50 thousand
E) 5 million

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
25
What happens to the half-life of a radioactive substance as it decays?
A) It decreases.
B) It remains constant.
C) It increases.
A) It decreases.
B) It remains constant.
C) It increases.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
26
The binding energy of most nuclei is proportional to
A)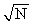
B) A2.
C) A3.
D) Z2.
E) A.
A)
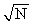
B) A2.
C) A3.
D) Z2.
E) A.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is NOT a nuclear "magic number"?
A) 8
B) 82
C) 28
D) 58
E) 20
A) 8
B) 82
C) 28
D) 58
E) 20

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
28
The binding energy per nucleon
A) is approximately constant throughout the periodic table, except for very light nuclei.
B) increases steadily as we go to heavier elements.
C) decreases steadily as we go to heavier elements.
D) has a minimum near iron in the periodic table.
A) is approximately constant throughout the periodic table, except for very light nuclei.
B) increases steadily as we go to heavier elements.
C) decreases steadily as we go to heavier elements.
D) has a minimum near iron in the periodic table.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
29
The neutron-proton stability curve of the isotopes of all elements will be plotted on a graph whose axes are shown in Figure 29-1.
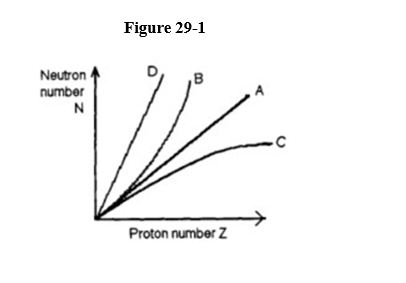
The plot will look like
A) graph A.
B) graph B.
C) graph C.
D) graph D.

The plot will look like
A) graph A.
B) graph B.
C) graph C.
D) graph D.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
30
Stable nuclei with mass numbers greater than 40 have
A) N > Z.
B) Z > N.
C) N > M.
D) M < N + Z.
E) M > N + Z.
A) N > Z.
B) Z > N.
C) N > M.
D) M < N + Z.
E) M > N + Z.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following combinations of nucleons would be the most likely to result in a stable nucleus? ("Odd" and "even" refer to the numbers of protons and neutrons in the nucleus.)
A) even protons, even neutrons
B) odd protons, odd neutrons
C) even protons, odd neutrons
D) odd protons, even neutrons
A) even protons, even neutrons
B) odd protons, odd neutrons
C) even protons, odd neutrons
D) odd protons, even neutrons

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
32
A roentgen (R) is
A) a dosage of 2.58 × 10-4 C/kg.
B) 3.70 × 1010 decays/s in radium.
C) the SI unit of absorbed dose = 1 J/kg = 100 rad.
D) the SI unit of radioactivity ( = 1 decay/s).
E) 10-2 J/kg.
A) a dosage of 2.58 × 10-4 C/kg.
B) 3.70 × 1010 decays/s in radium.
C) the SI unit of absorbed dose = 1 J/kg = 100 rad.
D) the SI unit of radioactivity ( = 1 decay/s).
E) 10-2 J/kg.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
33
A rad is
A) a dosage of 2.58 × 10-4 C/kg.
B) the SI unit of radioactivity ( = 1 decay/s).
C) 3.70 × 1010 decays/s in radium.
D) 10-2 J/kg.
E) the SI unit of absorbed dose = 1 J/kg = 100 rad.
A) a dosage of 2.58 × 10-4 C/kg.
B) the SI unit of radioactivity ( = 1 decay/s).
C) 3.70 × 1010 decays/s in radium.
D) 10-2 J/kg.
E) the SI unit of absorbed dose = 1 J/kg = 100 rad.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
34
A gray (Gy) is
A) the SI unit of radioactivity (= 1 decay/s).
B) 10-2 J/kg.
C) a dosage of 2.58 × 10-4 C/kg.
D) the SI unit of absorbed dose = 1 J/kg = 100 rad.
E) 3.70 × 1010 decays/s in radium.
A) the SI unit of radioactivity (= 1 decay/s).
B) 10-2 J/kg.
C) a dosage of 2.58 × 10-4 C/kg.
D) the SI unit of absorbed dose = 1 J/kg = 100 rad.
E) 3.70 × 1010 decays/s in radium.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
35
An RBE
A) when multiplied into dosage, yields the effective dose.
B) is an SI unit of absorbed dose = 1 J/kg = 100 rad.
C) is equivalent to 3.70 × 1010 decays/s in radium.
D) is an effective dose of 1 Gy.
E) is 10-2 J/kg.
A) when multiplied into dosage, yields the effective dose.
B) is an SI unit of absorbed dose = 1 J/kg = 100 rad.
C) is equivalent to 3.70 × 1010 decays/s in radium.
D) is an effective dose of 1 Gy.
E) is 10-2 J/kg.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
36
A sievert (Sv) is
A) the SI unit of absorbed dose = 1 J/kg = 100 rad.
B) 10-2 J/kg.
C) the effective dose of 1 Gy.
D) 3.70 × 1010 decays/s in radium.
E) a dosage of 2.58 × 10-4 C/kg.
A) the SI unit of absorbed dose = 1 J/kg = 100 rad.
B) 10-2 J/kg.
C) the effective dose of 1 Gy.
D) 3.70 × 1010 decays/s in radium.
E) a dosage of 2.58 × 10-4 C/kg.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
37
In which of the following application of technology in the home is radioactivity utilized?
A) a door-bell
B) a food sterilizer
C) a smoke detector
D) a humidifier
A) a door-bell
B) a food sterilizer
C) a smoke detector
D) a humidifier

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
38
A rem is
A) a dose that 3.70 × 1010 decays/s in radium.
B) produces same tissue damage as 1 rad.
C) the SI unit of absorbed dose = 1 J/kg = 100 rad.
D) a dosage of 2.58 × 10-4 C/kg.
E) an effective dose (= (rads)(RBE)).
A) a dose that 3.70 × 1010 decays/s in radium.
B) produces same tissue damage as 1 rad.
C) the SI unit of absorbed dose = 1 J/kg = 100 rad.
D) a dosage of 2.58 × 10-4 C/kg.
E) an effective dose (= (rads)(RBE)).

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
39
All of the following are units used to describe radiation dosage in humans except
A) Rad.
B) RBE.
C) rem.
D) Curie.
E) Sievert
A) Rad.
B) RBE.
C) rem.
D) Curie.
E) Sievert

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following have the least RBE (relative biological effectiveness)?
A) beta particles
B) slow neutrons
C) alpha particles
D) gamma rays
E) fast protons
A) beta particles
B) slow neutrons
C) alpha particles
D) gamma rays
E) fast protons

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
41
Donald Glazer
A) observed back-scattering from nuclei.
B) invented the particle detector tube.
C) invented the bubble chamber.
D) discovered radium and polonium.
E) discovered radioactivity.
A) observed back-scattering from nuclei.
B) invented the particle detector tube.
C) invented the bubble chamber.
D) discovered radium and polonium.
E) discovered radioactivity.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
42
The type of detector that uses liquid hydrogen is a
A) spark chamber.
B) cloud chamber.
C) Geiger tube.
D) bubble chamber.
E) scintillation counter.
A) spark chamber.
B) cloud chamber.
C) Geiger tube.
D) bubble chamber.
E) scintillation counter.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
43
Hans Geiger
A) discovered radium and polonium.
B) invented the bubble chamber.
C) discovered radioactivity.
D) observed back-scattering from nuclei.
E) invented the particle detector tube.
A) discovered radium and polonium.
B) invented the bubble chamber.
C) discovered radioactivity.
D) observed back-scattering from nuclei.
E) invented the particle detector tube.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
44
The type of detector that requires a magnetic field to "view" charged particles is a
A) Geiger tube.
B) spark chamber.
C) bubble chamber.
D) scintillation counter.
E) cloud chamber.
A) Geiger tube.
B) spark chamber.
C) bubble chamber.
D) scintillation counter.
E) cloud chamber.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
45
What are the mass number and the atomic number for each of the following:
(a) beta+?
(b) beta-?
(a) beta+?
(b) beta-?

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
46
Complete the following nuclear decay equation:



Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
47
Complete the following nuclear decay equation:



Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
48
Complete the following nuclear decay equation:
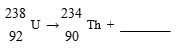
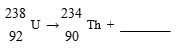

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
49
A nucleus M A N undergoes 1 alpha decay and 3 beta minus decay s.
(a) What is the resulting nucleus?
(b) If there had been beta plus decays, instead of beta minus decays, what would be the resulting nucleus?
(a) What is the resulting nucleus?
(b) If there had been beta plus decays, instead of beta minus decays, what would be the resulting nucleus?

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
50
Name the first three isotopes of hydrogen and their nuclide symbols, and indicate which is most common and which is most unstable.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
51
From a knowledge of the size and mass of a nucleus, one can estimate the density of nuclear material. Such a calculation shows that the density of nuclear matter is on the order of magnitude of
A) 104 kg/m3.
B) 1011 kg/m3.
C) 102 kg/m3.
D) 1017 kg/m3.
E) 108 kg/m3.
A) 104 kg/m3.
B) 1011 kg/m3.
C) 102 kg/m3.
D) 1017 kg/m3.
E) 108 kg/m3.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
52
How many protons are there in the carbon-14 nucleus?
A) 1
B) 14
C) 6
D) 8
E) none
A) 1
B) 14
C) 6
D) 8
E) none

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
53
If an element of atomic number 15 has an isotope of mass number 32
A) the number of protons in the nucleus is 17.
B) the number of neutrons in the nucleus is 15.
C) the number of protons in the nucleus is 32.
D) the number of nucleons in the nucleus is 15.
E) the number of neutrons in the nucleus is 17.
A) the number of protons in the nucleus is 17.
B) the number of neutrons in the nucleus is 15.
C) the number of protons in the nucleus is 32.
D) the number of nucleons in the nucleus is 15.
E) the number of neutrons in the nucleus is 17.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
54
Heavy-water molecules have a mass number of
A) 20.
B) 10.
C) 3.
D) 12.
E) 18.
A) 20.
B) 10.
C) 3.
D) 12.
E) 18.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
55
Which has the greater half-life?
A) Strontum-90
B) Cobalt-60
C) Uranium-238
D) Oxygen-19
E) Hydrogen-3
A) Strontum-90
B) Cobalt-60
C) Uranium-238
D) Oxygen-19
E) Hydrogen-3

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
56
A radioactive substance with a half-life of 3 days has an initial activity of 0.24 Ci. What is its activity after 6 days?
A) 0.12 Ci
B) 0.02 Ci
C) 0.06 Ci
D) 0.23 Ci
E) 0.48 Ci
A) 0.12 Ci
B) 0.02 Ci
C) 0.06 Ci
D) 0.23 Ci
E) 0.48 Ci

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
57
Plutonium has a half life of 2.4 × 104 years. How long does it take for 99% of the plutonium to decay?
A) 1.6 × 102 y
B) 1.6 × 104 y
C) 1.6 × 103 y
D) 1.6 × 106 y
E) 1.6 × 105 y
A) 1.6 × 102 y
B) 1.6 × 104 y
C) 1.6 × 103 y
D) 1.6 × 106 y
E) 1.6 × 105 y

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
58
The radioactivity due to Carbon-14 measured in a piece of a wooden casket from an ancient burial site was found to produce 20.0 counts per minute from a given sample, whereas the same amount of carbon from a piece of living wood produced 160. counts per minute. The half-life of Carbon-14, a beta emitter, is 5730. years. Thus we would estimate the age of the artifact to be about
A) 14,800 years
B) 17,200 years
C) 11,500 years
D) 20,000 years
E) 5,700 years
A) 14,800 years
B) 17,200 years
C) 11,500 years
D) 20,000 years
E) 5,700 years

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
59
Which isotope is used to define the ATOMIC MASS UNIT?
A) 1H
B) 2H
C) 4He
D) 16O
E) 12C
A) 1H
B) 2H
C) 4He
D) 16O
E) 12C

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following elements would be expected to be most stable?
A) Platinum-195
B) Helium-3
C) Iron-56
D) Bismuth-209
E) Carbon-12
A) Platinum-195
B) Helium-3
C) Iron-56
D) Bismuth-209
E) Carbon-12

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
61
Which is unstable?
A)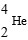
B)
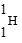
C)
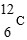
D)
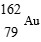
E)
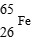
A)
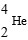
B)
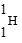
C)
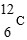
D)
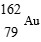
E)
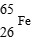

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
62
The binding energy of a 170Yb atom would be expected to be close to
A) 170. MeV.
B) 1.4 GeV.
C) 8. MeV.
D) 170. GeV.
E) 17. MeV.
A) 170. MeV.
B) 1.4 GeV.
C) 8. MeV.
D) 170. GeV.
E) 17. MeV.

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck
63
What is the binding energy of the 4He nucleus?
A) 10.77 MeV
B) 7.80 MeV
C) 14.15 MeV
D) 28.3 MeV
E) 20.36 MeV
A) 10.77 MeV
B) 7.80 MeV
C) 14.15 MeV
D) 28.3 MeV
E) 20.36 MeV

Unlock Deck
Unlock for access to all 63 flashcards in this deck.
Unlock Deck
k this deck


