Deck 27: Quantum Physics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/58
Play
Full screen (f)
Deck 27: Quantum Physics
1
Is the maximum kinetic energy of a photo-electron emitted from a metal in the photoelectric effect proportional to the frequency of the incident light?
Not unless the work function is zero. K = hf - φ so (K + φ) is proportional to frequency.
2
What was it that the Compton effect illustrated?
Electromagnetic waves (X-rays) could act as particles (photons) when scattering off electrons.
3
Which of Bohr's assumptions in creating the "Bohr atom" was revolutionary (unclassical)?
that angular momentum was quantized: mvr = n(h/2π); also radiates only when making transition to another orbit
4
How would Bohr's theory be changed if only gravitation held the electron to the nucleus?

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
5
Classical theory predicts that the planets can orbit the Sun quite stably. Why could not stable orbits be predicted for the atom by simply substituting the electrical attraction for the gravitational attraction? Why was there a classical crisis in describing the atom?

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
6
Give an example of a material whose useful property depends upon a metastable state.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
7
What are the two basic conditions necessary for laser action?

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
8
Planck's constant is
A) 6.6 × 1034 J-s.
B) E/ .
C) 4.1 × 10-15 eV-s.
D) 6.6 × 1028 MeV-s.
E) 4.1 × 10-15 eV-min.
A) 6.6 × 1034 J-s.
B) E/ .
C) 4.1 × 10-15 eV-s.
D) 6.6 × 1028 MeV-s.
E) 4.1 × 10-15 eV-min.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
9
The fact that mT = 2.9 × 10-3 m-K is known as
A) Planck's law.
B) Maxwell's law.
C) Wien's law.
D) Bohr's law.
E) de Broglie's law.
A) Planck's law.
B) Maxwell's law.
C) Wien's law.
D) Bohr's law.
E) de Broglie's law.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
10
The ultraviolet catastrophe is a catastrophe because
A) Wien's law predicts an infinite intensity of radiation at large wavelengths.
B) human vision cannot detect ultraviolet light directly.
C) Wien's law predicts an infinite intensity of radiation at small wavelengths.
D) ultraviolet light will not produce electrons by the photoelectric effect.
A) Wien's law predicts an infinite intensity of radiation at large wavelengths.
B) human vision cannot detect ultraviolet light directly.
C) Wien's law predicts an infinite intensity of radiation at small wavelengths.
D) ultraviolet light will not produce electrons by the photoelectric effect.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
11
A blackbody is an ideal system that
A) either absorbs 100% of the light incident upon it, or emits 100% of the radiation it generates.
B) absorbs 100% of the light incident upon it, but cannot emit light of its own (i.e., a "black" body).
C) absorbs 50% of the light incident upon it, and emits 50% of the radiation it generates.
D) emits 100% of the light it generates, but cannot absorb radiation of its own.
A) either absorbs 100% of the light incident upon it, or emits 100% of the radiation it generates.
B) absorbs 100% of the light incident upon it, but cannot emit light of its own (i.e., a "black" body).
C) absorbs 50% of the light incident upon it, and emits 50% of the radiation it generates.
D) emits 100% of the light it generates, but cannot absorb radiation of its own.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
12
You may have heard it said that all objects, even ones at the temperature of our bodies, are continually emitting electromagnetic radiation. Is this true?
A) Yes, and the radiation from our bodies is mostly in the infrared region of the spectrum, and hence not detected by our eyes.
B) Yes, and this radiation is mostly in the form of radio waves, which is a common source of static often heard on an AM radio.
C) No, this is not true. If a person were emitting electromagnetic radiation, he could be seen glowing in the dark, and this is obviously not the case for most people.
D) Yes, and the radiation referred to results from the trace amounts of radioactive material that have accumulated in our bodies from the food we eat.
A) Yes, and the radiation from our bodies is mostly in the infrared region of the spectrum, and hence not detected by our eyes.
B) Yes, and this radiation is mostly in the form of radio waves, which is a common source of static often heard on an AM radio.
C) No, this is not true. If a person were emitting electromagnetic radiation, he could be seen glowing in the dark, and this is obviously not the case for most people.
D) Yes, and the radiation referred to results from the trace amounts of radioactive material that have accumulated in our bodies from the food we eat.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
13
Albert Einstein received the 1921 Nobel Prize for his
A) Special Theory of Relativity.
B) Theory of the Photoelectric Effect.
C) Theory of the Brownian Motion.
D) General Theory of Relativity.
A) Special Theory of Relativity.
B) Theory of the Photoelectric Effect.
C) Theory of the Brownian Motion.
D) General Theory of Relativity.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
14
Classical theory predicted that the photocurrent, of the photoelectric effect, should be proportional to the
A) intensity of light.
B) electric field magnitude.
C) wavelength of light.
D) frequency of light.
A) intensity of light.
B) electric field magnitude.
C) wavelength of light.
D) frequency of light.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
15
In the photoelectric effect, the energies of the ejected electrons
A) vary randomly.
B) are proportional to the intensity of light.
C) are proportional to the frequency of light.
D) are proportional to the speed of light.
A) vary randomly.
B) are proportional to the intensity of light.
C) are proportional to the frequency of light.
D) are proportional to the speed of light.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
16
In a famous experiment done at the end of the 19th century, two metal electrodes were placed in an evacuated glass tube. A high voltage was applied between them. By using an appropriate metal for the cathode (e.g., sodium or potassium), it was found that a current could be made to flow when the cathode was illuminated with blue light, but no current would flow when red light was used, no matter how bright the light. Einstein was able to explain this strange phenomenon, and as a result, this experiment was considered the first experimental demonstration of
A) the particle nature of electrons.
B) the equivalency of electron and photons.
C) the wave nature of light.
D) the particle nature of light.
E) the wave nature of electrons.
A) the particle nature of electrons.
B) the equivalency of electron and photons.
C) the wave nature of light.
D) the particle nature of light.
E) the wave nature of electrons.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
17
________ the CUTOFF FREQUENCY, photons do not have enough energy to dislodge photoelectrons and no photocurrent is observed.
A) Below
B) Above
A) Below
B) Above

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
18
The photoelectric effect is explainable assuming
A) that light has a wave nature and a particle nature.
B) that light has a wave nature.
C) that light has a particle nature.
D) that light is an electromagnetic phenomena.
A) that light has a wave nature and a particle nature.
B) that light has a wave nature.
C) that light has a particle nature.
D) that light is an electromagnetic phenomena.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
19
In the photoelectric effect, the stopping potential is
A) e Kmax.
B) (Kmax)/e - https://storage.examlex.com/TB9720/https://storage.examlex.com/TB9720/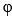 .
.
C) Kmax /e.
D) 11eea414_0aa8_cd77_97f4_af6d7fc69eef_TB9720_11.
E) Kmax.
A) e Kmax.
B) (Kmax)/e - https://storage.examlex.com/TB9720/https://storage.examlex.com/TB9720/
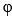 .
.C) Kmax /e.
D) 11eea414_0aa8_cd77_97f4_af6d7fc69eef_TB9720_11.
E) Kmax.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
20
The ratio of energy to frequency for a given photon gives
A) its work function.
B) Planck's constant.
C) its velocity.
D) its amplitude.
A) its work function.
B) Planck's constant.
C) its velocity.
D) its amplitude.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
21
Planck's constant
A) sets an upper limit to the amount of energy that can be absorbed or emitted.
B) sets a lower limit to the amount of energy that can be absorbed or emitted.
C) determines the value of the gravitational constant.
D) relates mass to energy.
A) sets an upper limit to the amount of energy that can be absorbed or emitted.
B) sets a lower limit to the amount of energy that can be absorbed or emitted.
C) determines the value of the gravitational constant.
D) relates mass to energy.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
22
The energy of a photon depends on
A) its frequency.
B) its velocity.
C) its amplitude.
A) its frequency.
B) its velocity.
C) its amplitude.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
23
Which color of light has the lowest energy photons?
A) yellow
B) green
C) red
D) blue
E) orange
A) yellow
B) green
C) red
D) blue
E) orange

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
24
What is a photon?
A) a small packet of electromagnetic energy that has particle-like properties
B) an electron in an excited state
C) an electron that has been made electrically neutral
D) one form of a nucleon, one of the particles that makes up the nucleus
A) a small packet of electromagnetic energy that has particle-like properties
B) an electron in an excited state
C) an electron that has been made electrically neutral
D) one form of a nucleon, one of the particles that makes up the nucleus

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
25
A photon is a particle that
A) has a velocity in a vacuum that varies with the photon frequency.
B) cannot travel in a vacuum.
C) has zero electric field associated with it.
D) has zero electric charge.
A) has a velocity in a vacuum that varies with the photon frequency.
B) cannot travel in a vacuum.
C) has zero electric field associated with it.
D) has zero electric charge.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is an accurate statement?
A) In vacuum, ultraviolet photons travel faster than infrared photons.
B) Infrared photons have enough energy to readily break bonds in a nucleic acid molecule like DNA.
C) Photons can have positive or negative charge.
D) An ultraviolet photon has more energy than does an infrared photon.
A) In vacuum, ultraviolet photons travel faster than infrared photons.
B) Infrared photons have enough energy to readily break bonds in a nucleic acid molecule like DNA.
C) Photons can have positive or negative charge.
D) An ultraviolet photon has more energy than does an infrared photon.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
27
In order for a photon to eject an electron from a metal's surface in the photoelectric effect, the photon's
A) momentum must be zero.
B) wavelength must be greater than a certain minimum value.
C) frequency must be greater than a certain minimum value.
D) speed must be greater than a certain minimum value.
A) momentum must be zero.
B) wavelength must be greater than a certain minimum value.
C) frequency must be greater than a certain minimum value.
D) speed must be greater than a certain minimum value.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
28
When the accelerating voltage in an X-ray tube is doubled, the minimum wavelength of the X-rays
A) is decreased to one-fourth the original value.
B) is decreased to one-half the original value.
C) is increased to four times the original value.
D) is increased to twice the original value.
A) is decreased to one-fourth the original value.
B) is decreased to one-half the original value.
C) is increased to four times the original value.
D) is increased to twice the original value.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
29
When monochromatic X-rays are scattered by a material, the wavelength of the scattered radiation does not depend upon
A) the scattering angle.
B) the type of atom.
C) incident wavelength.
D) mass of the interacting particle.
A) the scattering angle.
B) the type of atom.
C) incident wavelength.
D) mass of the interacting particle.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
30
The Compton effect importantly demonstrated which property of electromagnetic radiation?
A) momenta
B) its wavelength
C) particle nature
D) energy content
A) momenta
B) its wavelength
C) particle nature
D) energy content

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
31
In the Compton effect, as the scattering angle increases, the frequency of the X-rays scattered at that angle
A) varies randomly.
B) decreases.
C) does not change.
D) increases.
A) varies randomly.
B) decreases.
C) does not change.
D) increases.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
32
In a Compton scattering experiment, what scattering angle produces the greatest change in wavelength?
A) 45.°
B) 180.°
C) zero degrees
D) 90.°
E) 270.°
A) 45.°
B) 180.°
C) zero degrees
D) 90.°
E) 270.°

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
33
Bound electrons have
A) infinite energy.
B) zero energy.
C) imaginary energy.
D) positive energy.
E) negative energy.
A) infinite energy.
B) zero energy.
C) imaginary energy.
D) positive energy.
E) negative energy.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
34
Photons are emitted when a bound electron jumps up to a(n) ________ energy state.
A) zero
B) higher
C) lower
D) infinite
A) zero
B) higher
C) lower
D) infinite

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
35
Light from a gas discharge tube gives a (an) ________ spectrum.
A) emission
B) blackbody
C) continuous
D) absorption
A) emission
B) blackbody
C) continuous
D) absorption

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
36
Which hydrogen spectral series falls in the visible region?
A) Lyman
B) Paschen
C) Balmer
D) Brackett
A) Lyman
B) Paschen
C) Balmer
D) Brackett

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
37
The energy difference between adjacent orbit radii in a hydrogen atom
A) remains constant for all values of n.
B) increases with increasing values of n.
C) varies randomly with increasing values of n.
D) decreases with increasing values of n.
A) remains constant for all values of n.
B) increases with increasing values of n.
C) varies randomly with increasing values of n.
D) decreases with increasing values of n.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
38
When an electron jumps from an orbit where n = 4 to one where n = 3
A) two photons are emitted.
B) a photon is emitted.
C) a photon is absorbed.
D) two photons are absorbed.
A) two photons are emitted.
B) a photon is emitted.
C) a photon is absorbed.
D) two photons are absorbed.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
39
In order for the atoms in a neon discharge tube (or a neon beer sign) to emit their characteristic orange light, it is necessary that
A) the atoms be continually replaced with fresh atoms, because the energy of the atoms tends to be used up with continued excitation, resulting in dimmer and dimmer light.
B) electrons first be given energy to raise them from their ground state to an excited state.
C) there be no unoccupied energy levels in each atom.
D) each atom carry a net electric charge.
A) the atoms be continually replaced with fresh atoms, because the energy of the atoms tends to be used up with continued excitation, resulting in dimmer and dimmer light.
B) electrons first be given energy to raise them from their ground state to an excited state.
C) there be no unoccupied energy levels in each atom.
D) each atom carry a net electric charge.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
40
In the Bohr theory the orbital radius depends upon the principal quantum number in what way?
A) n3
B) n
C) 1/n
D) n2
E) 1/n2
A) n3
B) n
C) 1/n
D) n2
E) 1/n2

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
41
How many of the infinite number of Balmer spectrum lines are in the visible spectrum range?
A) none
B) 2
C) 4
D) 6
E) infinite
A) none
B) 2
C) 4
D) 6
E) infinite

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
42
The word LASER is an acronym for
A) Light Amplification by the Stimulated Emission of Radiation.
B) Light Altered Spectra of Energy Radiated.
C) Light Absorbed States of Energetic Resonance.
D) LAtent Source of Enhanced Radiation.
A) Light Amplification by the Stimulated Emission of Radiation.
B) Light Altered Spectra of Energy Radiated.
C) Light Absorbed States of Energetic Resonance.
D) LAtent Source of Enhanced Radiation.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
43
In a He-Ne laser, the actual emission line at 632.8 nm comes from a jump down in what atom(s)?
A) helium
B) oxygen
C) hydrogen
D) neon
E) helium and neon
A) helium
B) oxygen
C) hydrogen
D) neon
E) helium and neon

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
44
The compact disc stores information by means of
A) Compton scattering.
B) stimulated absorption.
C) pits and lands.
D) the photoelectric effect.
E) holography.
A) Compton scattering.
B) stimulated absorption.
C) pits and lands.
D) the photoelectric effect.
E) holography.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
45
When a hologram is illuminated with a beam of coherent light, it produces
A) both a real and a virtual image.
B) only a virtual image of the object.
C) only a real image of the object.
A) both a real and a virtual image.
B) only a virtual image of the object.
C) only a real image of the object.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
46
The human eye can just detect green light of wavelength 500. nm, which arrives at the retina at the rate of 2 × 10-18 W. How many photons arrive each second?

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
47
The graph shown in Figure 27-1 is a plot based on student data from their testing of a
photoelectric material.
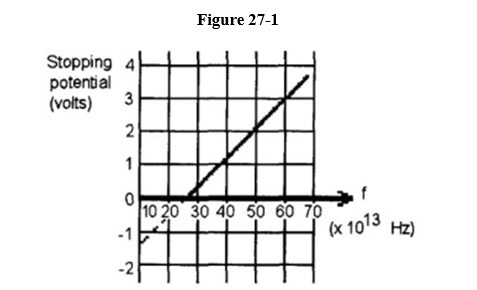
(a) Determine the cutoff frequency.
(b) Determine the work function.
photoelectric material.

(a) Determine the cutoff frequency.
(b) Determine the work function.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
48
What is the radius of the electron orbit in a singly ionized helium ion?

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
49
What is the ionization energy for a doubly ionized lithium ion (Z = 3)?

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
50
What is the energy, in eV, of the n = 3 state in hydrogen?

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
51
Bohr's atomic theory predicted energy levels for hydrogen of En = -(2 π2k2e4m) about a bare nucleus of atomic number Z, how do you expect Z to be incorporated into the En formula and why?

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
52
If sunlight peaks at 0.55 ?m wavelength, this infers a surface temperature of the sun of how many thousand degrees Kelvin?
A) 2.5
B) 9.5
C) 5.3
D) 15.
E) 25.
A) 2.5
B) 9.5
C) 5.3
D) 15.
E) 25.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
53
How much energy is carried by a photon of wavelength 660. nm?
A) 4.78 × 10-19 eV
B) 6.63 × 10-34 J
C) 5.39 eV
D) 3.01 × 10-19 J
E) 1.46 × 10-48 J
A) 4.78 × 10-19 eV
B) 6.63 × 10-34 J
C) 5.39 eV
D) 3.01 × 10-19 J
E) 1.46 × 10-48 J

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
54
What change in wavelength is expected when X-rays are scattered at 90°?
A) 3.75 × 10-3 nm
B) 4.84 × 10-3 nm
C) 2.42 × 10-3 nm
D) 1.18 × 10-3 nm
E) no change at this angle
A) 3.75 × 10-3 nm
B) 4.84 × 10-3 nm
C) 2.42 × 10-3 nm
D) 1.18 × 10-3 nm
E) no change at this angle

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
55
The energy required to ionize a hydrogen atom from the ground state is
A) 3.4 eV.
B) 6.8 eV.
C) 13.6 eV.
D) infinite.
E) 27.2 eV.
A) 3.4 eV.
B) 6.8 eV.
C) 13.6 eV.
D) infinite.
E) 27.2 eV.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
56
Consider an atom with four accessible energy levels. What is the maximum number of different wavelengths that could be emitted by such an atom?
A) 5
B) 8
C) 4
D) 6
E) 7
A) 5
B) 8
C) 4
D) 6
E) 7

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
57
In state n = 1, the energy of the hydrogen atom is -13.58 eV. What is its energy in state n = 2?
A) -1.51 eV
B) -6.79 eV
C) -4.53 eV
D) -5.89 eV
E) -3.40 eV
A) -1.51 eV
B) -6.79 eV
C) -4.53 eV
D) -5.89 eV
E) -3.40 eV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
58
What is the ionization energy of singly ionized helium?
A) 54.4 eV
B) 27.2 eV
C) 6.80 eV
D) 13.6 eV
E) 36.4 eV
A) 54.4 eV
B) 27.2 eV
C) 6.80 eV
D) 13.6 eV
E) 36.4 eV

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck


