Deck 10: Temperature and Kinetic Theory
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/68
Play
Full screen (f)
Deck 10: Temperature and Kinetic Theory
1
Some texts use the kilogram-mole rather than the gram-mole. What would be the values of the gas constant R and Avogadro's number NA using the kg-mole?
R = 8.31 × 103 J / [(kg-mole) K]
NA = 6.02 × 1026 (kg-mole)-1
NA = 6.02 × 1026 (kg-mole)-1
2
State the equipartition theorem.
On average, the total internal energy of an ideal gas is divided equally among each degree of freedom its molecules possesses. Futhermore, each degree of freedom contributes 1/2 NkBT to the total internal energy of the gas.
3
9/5 of a Fahrenheit degree is equivalent to
A) 1° C.
B) 1 K°.
C) 373.16 K.
D) 1 C°.
E) 273.16 K.
A) 1° C.
B) 1 K°.
C) 373.16 K.
D) 1 C°.
E) 273.16 K.
1 C°.
4
Boyle's law and Charles' law each respectively assume what is held constant?
A) temperature, volume
B) volume, pressure
C) pressure, volume
D) temperature, pressure
E) volume, temperature
A) temperature, volume
B) volume, pressure
C) pressure, volume
D) temperature, pressure
E) volume, temperature

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
5
The universal gas constant has what units?
A) molecules/mole
B) Joules
C) Joules/K
D) 1/C°
E) J/mol-K
A) molecules/mole
B) Joules
C) Joules/K
D) 1/C°
E) J/mol-K

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
6
Avogadro's number has what units?
A) J/mol-K
B) It's dimensionless.
C) molecules/mole
D) 1/C°
E) joules/mole
A) J/mol-K
B) It's dimensionless.
C) molecules/mole
D) 1/C°
E) joules/mole

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
7
Which temperature scale never gives negative temperatures?
A) Celsius
B) Kelvin
C) Fahrenheit
A) Celsius
B) Kelvin
C) Fahrenheit

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
8
Room temperature is approximately
A) -68.° F.
B) 68. K.
C) 300.° F.
D) 300. K.
E) 68.° C.
A) -68.° F.
B) 68. K.
C) 300.° F.
D) 300. K.
E) 68.° C.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
9
The Temperature of triple point of water is equivalent to which of the following?
A) 100.° C
B) 0.° F
C) 459.° F
D) 373.16 K
E) 273.16 K
A) 100.° C
B) 0.° F
C) 459.° F
D) 373.16 K
E) 273.16 K

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
10
The boiling point of water is at
A) -459. K.
B) 212.° C.
C) 373.16 K.
D) 459.° F.
E) 273.16 K.
A) -459. K.
B) 212.° C.
C) 373.16 K.
D) 459.° F.
E) 273.16 K.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
11
If the pressure acting on an ideal gas at constant temperature is tripled, its volume is
A) reduced to one-half.
B) reduced to one-third.
C) increased by a factor of two.
D) increased by a factor of three.
A) reduced to one-half.
B) reduced to one-third.
C) increased by a factor of two.
D) increased by a factor of three.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
12
Both the pressure and volume of a given sample of an ideal gas double. This means that its temperature in Kelvins must
A) double.
B) remain unchanged.
C) reduce to one-half its original value.
D) reduce to one-fourth its original value.
E) quadruple.
A) double.
B) remain unchanged.
C) reduce to one-half its original value.
D) reduce to one-fourth its original value.
E) quadruple.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following would correctly convert a Fahrenheit temperature (F) to a Kelvin (K) value?
A) K = (5/9)F + 255
B) K = (5/9)F - 273
C) K = (9/5) F + 273
D) K = (5/9)(F - 32)
E) K = (9/5)(F - 32) + 273
A) K = (5/9)F + 255
B) K = (5/9)F - 273
C) K = (9/5) F + 273
D) K = (5/9)(F - 32)
E) K = (9/5)(F - 32) + 273

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
14
When the engine of your car heats up, the spark plug gap will
A) decrease at first and then increase later, so that the two effects cancel once the engine reaches operating temperature.
B) decrease.
C) increase.
D) remain unchanged.
A) decrease at first and then increase later, so that the two effects cancel once the engine reaches operating temperature.
B) decrease.
C) increase.
D) remain unchanged.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
15
Aluminum has a positive coefficient of thermal expansion. Consider a round hole that has been drilled in a large sheet of aluminum. As the temperature increases and the surrounding metal expands, the hole diameter will
A) depends how much metal surrounds the hole
B) decrease.
C) remain constant.
D) increase.
A) depends how much metal surrounds the hole
B) decrease.
C) remain constant.
D) increase.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
16
The surface water temperature on a large, deep lake is 3° C. A sensitive temperature probe is lowered several m into the lake. What temperature will the probe record?
A) a temperature less than 3° C
B) a temperature equal to 3° C
C) a temperature warmer than 3° C
A) a temperature less than 3° C
B) a temperature equal to 3° C
C) a temperature warmer than 3° C

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
17
Due to the unusual thermal expansion properties of water, when a lake freezes during the winter, the water at the bottom of the lake is at ________ temperature than the water above it.
A) a lower
B) the same
C) a higher
A) a lower
B) the same
C) a higher

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
18
Water has its ________ density at 4.° C.
A) maximum
B) minimum
C) average
A) maximum
B) minimum
C) average

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
19
Consider a flat steel plate with a hole through its center. When the plate's temperature is decreased, the hole will
A) contract only if it takes up more than half the plate's surface area.
B) expand.
C) contract.
D) expand if it takes up less than half the plate's surface area.
A) contract only if it takes up more than half the plate's surface area.
B) expand.
C) contract.
D) expand if it takes up less than half the plate's surface area.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
20
A bimetallic strip, consisting of metal G on the top and metal H on the bottom, is rigidly attached to a wall at the left, as shown in Figure 10-1. The coefficient of linear thermal expansion for metal G is greater than that of metal H.
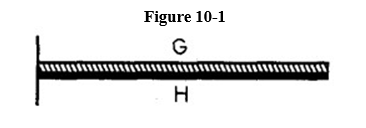
If the strip is uniformly heated, it will
A) curve downward.
B) remain horizontal, but get longer.
C) curve upward.
D) bend in the middle.

If the strip is uniformly heated, it will
A) curve downward.
B) remain horizontal, but get longer.
C) curve upward.
D) bend in the middle.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
21
In the equation PV = NkT, the k is known as
A) the spring (compressibility) constant.
B) compressibility.
C) Planck's constant.
D) Boltzmann's constant.
E) Avogadro's number.
A) the spring (compressibility) constant.
B) compressibility.
C) Planck's constant.
D) Boltzmann's constant.
E) Avogadro's number.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
22
Boltzmann's constant has what units?
A) molecules/mole
B) J/K
C) Joules/mole
D) 1/C°
E) J/mol-K
A) molecules/mole
B) J/K
C) Joules/mole
D) 1/C°
E) J/mol-K

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
23
Internal energy has what units?
A) 1/C°
B) J/mol-K
C) J/K
D) molecules/mole
E) J
A) 1/C°
B) J/mol-K
C) J/K
D) molecules/mole
E) J

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
24
The kinetic theory of gases suggests the average kinetic energy per molecule is (for a monatomic gas)
A) 3/2 kT.
B) 1/2 kT.
C) kT.
D) 5/2 kT.
E) 2 kT.
A) 3/2 kT.
B) 1/2 kT.
C) kT.
D) 5/2 kT.
E) 2 kT.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
25
Diffusion is described by
A) Graham's law.
B) Boyle's law.
C) Archimedes' law.
D) Kepler's law.
E) Charles' law.
A) Graham's law.
B) Boyle's law.
C) Archimedes' law.
D) Kepler's law.
E) Charles' law.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
26
Gresham's Law says, at constant pressure and temperature, the diffusion rate for a gas molecule is
A) inversely proportional to mass.
B) proportional to the square root of the mass.
C) inversely proportional to the square root of the mass.
D) proportional to mass.
A) inversely proportional to mass.
B) proportional to the square root of the mass.
C) inversely proportional to the square root of the mass.
D) proportional to mass.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
27
In order to double the average speed of the molecules in a given sample of gas, the temperature (measured in Kelvins) must
A) quadruple.
B) double.
C) reduce to one-fourth its original value.
D) triple.
E) reduce to one-half its original value.
A) quadruple.
B) double.
C) reduce to one-fourth its original value.
D) triple.
E) reduce to one-half its original value.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
28
An ideal gas at STP is first compressed until its volume is half the initial volume, and then it is allowed to expand until its pressure is half the initial pressure. All of this is done while holding the temperature constant. If the initial internal energy of the gas is U, the final internal energy of the gas will be
A) 2U.
B) U.
C) U/2.
D) U/3.
E) U/4.
A) 2U.
B) U.
C) U/2.
D) U/3.
E) U/4.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
29
A sample of an ideal gas is slowly compressed to one-half its original volume with no change in temperature. What happens to the average speed of the molecules in the sample?
A) It does not change.
B) It halves.
C) It doubles.
D) cannot be determined without more information
A) It does not change.
B) It halves.
C) It doubles.
D) cannot be determined without more information

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
30
A sample of an ideal gas is heated and its Kelvin temperature doubles. What happens to the average speed of the molecules in the sample?
A) it is 1/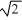 times smaller.
times smaller.
B) It doubles.
C) It halves.
D) it is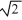 times as large.
times as large.
E) It does not change.
A) it is 1/
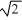 times smaller.
times smaller.B) It doubles.
C) It halves.
D) it is
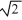 times as large.
times as large.E) It does not change.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
31
Oxygen molecules are 16 times more massive than hydrogen molecules. At a given temperature, how do their average molecular speeds compare? The oxygen molecules are moving
A) at 1/4 the speed.
B) 16 times faster.
C) 4 times faster.
D) the same as the hydrogen molecules.
E) at 1/16 the speed.
A) at 1/4 the speed.
B) 16 times faster.
C) 4 times faster.
D) the same as the hydrogen molecules.
E) at 1/16 the speed.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
32
Oxygen molecules are 16 times more massive than hydrogen molecules. At a given temperature, the average molecular kinetic energy of oxygen, compared to hydrogen,
A) is less.
B) is greater.
C) cannot be determined since pressure and volume are not given.
D) is the same.
A) is less.
B) is greater.
C) cannot be determined since pressure and volume are not given.
D) is the same.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
33
A container is filled with a mixture of helium and oxygen gases. A thermometer in the container indicates that the temperature is 22.° C. Which gas molecules have the greater average kinetic energy?
A) It is the same for both because the temperatures are the same.
B) The oxygen molecules do because they are diatomic.
C) The oxygen molecules do because they are more massive.
D) The helium molecules do because they are less massive.
E) The helium molecules do because they are monatomic.
A) It is the same for both because the temperatures are the same.
B) The oxygen molecules do because they are diatomic.
C) The oxygen molecules do because they are more massive.
D) The helium molecules do because they are less massive.
E) The helium molecules do because they are monatomic.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
34
What is the average separation between air molecules at STP?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
35
A constant pressure gas thermometer is initially at 28.° C. If the volume of gas increases by 10%, what is the final Celsius temperature?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
36
A container of an ideal gas at 1 atm is compressed to one-third its volume, with the temperature held constant. What is its final pressure?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
37
"Absolute Zero" is how many Fahrenheit degrees below freezing (water)?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
38
Two liters of a perfect gas are at 0° C and 1.0 atm. If the gas is nitrogen, N2, determine:
(a) the number of mol;
(b) the number of molecules; and
(c) the mass of the gas.
(a) the number of mol;
(b) the number of molecules; and
(c) the mass of the gas.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
39
Consider a classroom 10.0 m long by 6.0 m wide by 4.0 m high at "room temperature" 20.° C and 1.0 atmosphere.
(a) How many moles of gas are contained in the room?
(b) Assuming an average molecular weight of 28, what is the mass of the room air?
(a) How many moles of gas are contained in the room?
(b) Assuming an average molecular weight of 28, what is the mass of the room air?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
40
The coefficient of linear expansion for aluminum is 24. × 10-6 K-1. What is the coefficient in units of C °-1?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
41
Ten meter long steel railroad rails are laid end to end, with no space between, on a hot day when the temperature is 115.° F. Six months later the temperature has dropped to -2.°F. How much space now exists between each rail?
[ linear expansion coef. for steel is 12. × 10-6 C °-1]
[ linear expansion coef. for steel is 12. × 10-6 C °-1]

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
42
Consider an aluminum rod encircling the Earth (radius 6378. km). If the linear thermal expansion coefficient of the aluminum is 24. × 10-6 (C °)-1, and if the temperature of the rod increases by 1.0 C°, how high off the ground would the expanded ring be (assuming uniform space all around)?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
43
The thermal coefficient of linear expansion of Copper is 1.7 × 10-5 C °-1. What is the thermal volume expansion coefficient?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
44
A mercury thermometer has a bulb of volume 0.100 cm3 at 10.°C. The capillary tube above the bulb has a cross-sectional area of 0.012 mm2. The volume thermal expansion coefficient of mercury is 1.80 × 10-4 K-1. How much will the mercury rise when the temperature rises to 30.° C?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
45
At room temperature (20.° C) the area of a hole in a sheet of material is 5.505 cm2. When heated to 830.° C the area increases by 29.0 mm2.
(a) What is the thermal coefficient of linear expansion for the material?
(b) If the temperature had decreased to -76.° Fahrenheit, what would be the area of the hole?
(a) What is the thermal coefficient of linear expansion for the material?
(b) If the temperature had decreased to -76.° Fahrenheit, what would be the area of the hole?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
46
Given the following four speeds (in m/s) 16.0, 22.0, 36.0, and 38.0:
(a) what is the average speed?
(b) what is the rms speed?
(a) what is the average speed?
(b) what is the rms speed?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
47
The temperature is such that the rms speed of nitrogen molecules is 900. m/s at 750. mm Hg pressure. If the pressure increases to 780. mm Hg while the temperature remains constant, what is the rms speed now? [Molecular weight of nitrogen molecule is 28.]

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
48
Heat is added to an ideal gas at 20.° C. If the internal energy of the gas increases by a factor of three during this process, what is the final temperature?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
49
At what temperature is the average kinetic energy of an atom in helium gas equal to 6.21 × 10-21 J?

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
50
A mixture of helium and another gas diffuses through a porous barrier. The helium diffuses 3.16 times faster than the other type molecule. Identify the other gas.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
51
How many moles are there in 2.0 kg of copper? (The atomic weight of copper is 63.5 and its specific gravity is 8.9.)

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
52
Express your body temperature (98.6°)
A) 45.5° C
B) 29.5° C
C) 66.6° C
D) 37.0° C
E) 72.6° C
F) in Celsius degrees.
A) 45.5° C
B) 29.5° C
C) 66.6° C
D) 37.0° C
E) 72.6° C
F) in Celsius degrees.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
53
Express 45° C in °F.
A) 127° F
B) 81° F
C) 57° F
D) 113° F
E) 25° F
A) 127° F
B) 81° F
C) 57° F
D) 113° F
E) 25° F

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
54
A temperature change of 20 C° corresponds to a temperature change of
A) 11.1 F°.
B) 68. F°.
C) 36. F°.
D) 42. F°.
E) 20. F°.
A) 11.1 F°.
B) 68. F°.
C) 36. F°.
D) 42. F°.
E) 20. F°.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
55
"Absolute Zero" is what temperature on the Celsius and Fahrenheit scales respectively?
A) 0° C, 459° F
B) -273° C, -212° F
C) -212° C, -273° F
D) -273° C, -459° F
E) -459° C, -273° F
A) 0° C, 459° F
B) -273° C, -212° F
C) -212° C, -273° F
D) -273° C, -459° F
E) -459° C, -273° F

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
56
Which two temperature changes are equivalent?
A) -40 C°, -40 F°
B) 1 K, 1 F°
C) 1 C°, 1 K
D) 1 F°, 1 C°
A) -40 C°, -40 F°
B) 1 K, 1 F°
C) 1 C°, 1 K
D) 1 F°, 1 C°

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
57
On a winter -3.° C morning, the pressure in a car tire reads 28. psi on a pressure gauge. After several hours of highway traveling, the pressure reads 35. psi. Assuming the volume remains constant and normal atmospheric pressure of 15. psi, what is the tire temperature?
A) 45° C
B) 25° C
C) 41° C
D) 55° C
E) 55° F
A) 45° C
B) 25° C
C) 41° C
D) 55° C
E) 55° F

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
58
A sample of a diatomic ideal gas occupies 33.6 L under standard conditions. How many moles of gas are in the sample?
A) 2.0
B) 3.0
C) 4.5
D) 1.5
E) 0.75
A) 2.0
B) 3.0
C) 4.5
D) 1.5
E) 0.75

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
59
The temperature of an ideal gas increases from 10° C to 300. K while remaining at constant pressure. What happens to the volume of the gas?
A) It more than doubles.
B) It decreases slightly.
C) It increases by factor of 30.
D) It decreases to one-half its original volume.
E) It increases slightly.
A) It more than doubles.
B) It decreases slightly.
C) It increases by factor of 30.
D) It decreases to one-half its original volume.
E) It increases slightly.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
60
Consider an ideal gas at 1.0 atmosphere in a closed container. If the temperature changes from 300. K to 450. K, the final gauge pressure will be
A) 21. psi.
B) 0.5 atm.
C) 3.0 atm.
D) 4.5 atm.
E) 1.5 atm.
A) 21. psi.
B) 0.5 atm.
C) 3.0 atm.
D) 4.5 atm.
E) 1.5 atm.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
61
How many water molecules are there in 36. g of water? Express your answer as a multiple of Avogadro's number NA. (The molecular structure of a water molecule is H2O.)
A) 0.020 NA
B) 18. NA
C) 36. NA
D) 2.0 NA
E) 3.0 NA
A) 0.020 NA
B) 18. NA
C) 36. NA
D) 2.0 NA
E) 3.0 NA

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
62
By how much will a slab of concrete 18. m long contract when the temperature drops from 24.° C to -16.° C? (The coefficient of linear thermal expansion for concrete is 12 × 10-6 per degree C.)
A) 70. mm
B) 1.5 cm
C) 15. cm
D) 0.5 cm
E) 0.86 cm
A) 70. mm
B) 1.5 cm
C) 15. cm
D) 0.5 cm
E) 0.86 cm

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
63
A bolt hole in a brass plate has a diameter of 1.2 cm at 20.° C. What is the diameter of the hole when the plate is heated to 220.° C? (The coefficient of linear thermal expansion for brass is 19 × 10-6 per degree C.)
A) 1.200 cm
B) 1.190 cm
C) 1.210 cm
D) 1.195 cm
E) 1.205 cm
A) 1.200 cm
B) 1.190 cm
C) 1.210 cm
D) 1.195 cm
E) 1.205 cm

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
64
The coefficient of linear expansion for brass is 19. × 10-6 K-1 . What is its coefficient of volume expansion?
A) 3.8 × 10-6 K-1
B) 5.7 × 10-5 K-1
C) 8 × 10-18 K-1
D) 57. × 10-5 K-1
E) 38. × 10-6 K-1
A) 3.8 × 10-6 K-1
B) 5.7 × 10-5 K-1
C) 8 × 10-18 K-1
D) 57. × 10-5 K-1
E) 38. × 10-6 K-1

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
65
The volume coefficient of thermal expansion for gasoline is 9.50 × 10-4 per degree C. By how much does the volume of 1 L of gasoline change when the temperature rises from 20.°C to 40.°C?
A) 12. cm3
B) 6.0 cm3
C) 37. cm3
D) 24. cm3
E) 19. cm3
A) 12. cm3
B) 6.0 cm3
C) 37. cm3
D) 24. cm3
E) 19. cm3

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
66
How much compressional stress builds up in a thick aluminum bar held between 2 immovable walls if the temperature increases by 20. C°?
( = 24. × 10-6 C-1, Y = 7 × 1010 N/m2)
A) 34. × 107 N/m2
B) 34. × 108 N/m2
C) 34. × 106 N/m2
D) 34. × 104 N/m2
E) 34. × 105 N/m2
( = 24. × 10-6 C-1, Y = 7 × 1010 N/m2)
A) 34. × 107 N/m2
B) 34. × 108 N/m2
C) 34. × 106 N/m2
D) 34. × 104 N/m2
E) 34. × 105 N/m2

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
67
A confined gas has an average energy/molecule of 60. meV. If the pressure is tripled and the volume is reduced by a factor of 3, what now is the average energy?
A) 0.18 eV
B) 0.10 eV
C) 0.03 eV
D) 0.06 eV
E) 0.02 eV
A) 0.18 eV
B) 0.10 eV
C) 0.03 eV
D) 0.06 eV
E) 0.02 eV

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
68
At which of the following temperatures would we expect gas molecules to have half the energy that they have at 100.° C?
A) -86.° C
B) 93. K
C) 323. K
D) -56.° C
E) 50.° C
A) -86.° C
B) 93. K
C) 323. K
D) -56.° C
E) 50.° C

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck


