Deck 1: The Chemical Context of Life, Population Ecology, Genomes and Their Evolution
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/25
Play
Full screen (f)
Deck 1: The Chemical Context of Life, Population Ecology, Genomes and Their Evolution
1
If an atom of sulfur (atomic number 16) were allowed to react with atoms of hydrogen (atomic number 1), which of the molecules below would be formed?
A) S-H
B) H-S-H
C)
D)
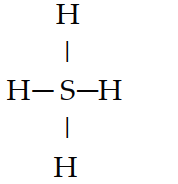
E) H=S=H
A) S-H
B) H-S-H
C)
D)
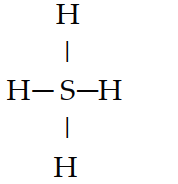
E) H=S=H
H-S-H
2
In ammonium chloride salt (NH4 Cl) the anion is a single chloride ion, Cl-. What is the cation of NH4 Cl?
A) N, with a charge of +3
B) H, with a charge of +1
C) H2 with a charge of +4
D) NH4 with a charge of +1
E) NH4 with a charge of +4
A) N, with a charge of +3
B) H, with a charge of +1
C) H2 with a charge of +4
D) NH4 with a charge of +1
E) NH4 with a charge of +4
NH4 with a charge of +1
3
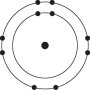
Which one of the atoms shown would be most likely to form a cation with a charge of +1?
A)
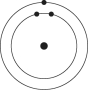
B)
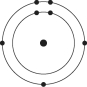
C)
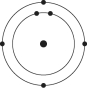
D)
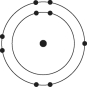
E)
A)

B)

C)

D)

E)


4
Which one of the atoms shown would be most likely to form an anion with a charge of -1?
A)
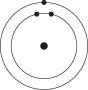
B)
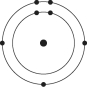
C)
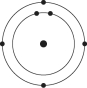
D)
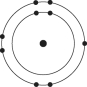
E)
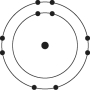
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following pairs of atoms would be most likely to form a covalent bond?
A)

B)

C)

D)

E)

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following pairs of atoms would be most likely to form an ionic bond?
A)

B)

C)

D)

E)

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
7
A given solution contains 0.0001(10-4) moles of hydrogen ions [H+] per liter. Which of the following best describes this solution?
A) acidic: H+ acceptor
B) basic: H+ acceptor
C) acidic: H+ donor
D) basic: H+ donor
E) neutral
A) acidic: H+ acceptor
B) basic: H+ acceptor
C) acidic: H+ donor
D) basic: H+ donor
E) neutral

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
8
Carbon dioxide (CO2) is readily soluble in water, according to the equation CO2 + H2O H2CO3. Carbonic acid (H2CO3) is a weak acid. If CO2 is bubbled into a beaker containing pure, freshly-distilled water, which of the following graphs correctly describes the results?
A)
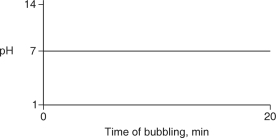
B)
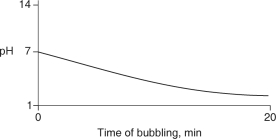
C)
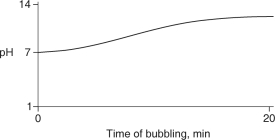
D)
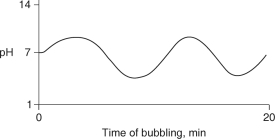
E)
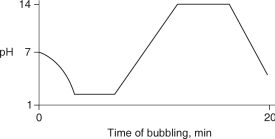
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
9
Carbon dioxide (CO2) is readily soluble in water, according to the equation CO2 + H2O H2CO3. Carbonic acid (H2CO3) is a weak acid. Respiring cells release CO2. What prediction can we make about the pH of blood as that blood first comes in contact with respiring cells?
A) Blood pH will decrease slightly.
B) Blood pH will increase slightly.
C) Blood pH will remain unchanged.
D) Blood pH will first increase, then decrease as CO2 combines with hemoglobin
E)Blood pH will first decrease, then increase sharply as CO2 combines with hemoglobin.
A) Blood pH will decrease slightly.
B) Blood pH will increase slightly.
C) Blood pH will remain unchanged.
D) Blood pH will first increase, then decrease as CO2 combines with hemoglobin
E)Blood pH will first decrease, then increase sharply as CO2 combines with hemoglobin.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the pairs of molecular structures shown below depict enantiomers (enantiomeric forms) of the same molecule?
A)
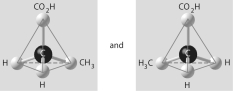
B)
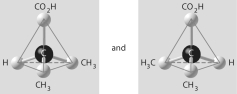
C)
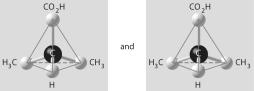
D)
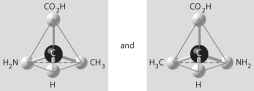
E)

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the pairs of molecular structures shown below do NOT depict enantiomers (enantiomeric forms) of the same molecule?
A)
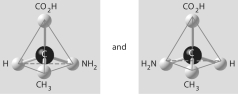
B)
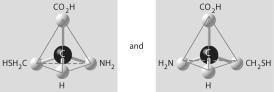
C)
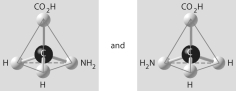
D)
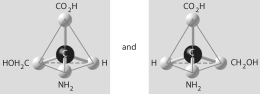
E)
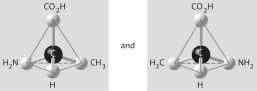
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
12
Identify the asymmetric carbon in this molecule:
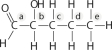
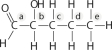

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
13
For the following questions, match the labeled component of the cell membrane (Figure 7.1) with its description.
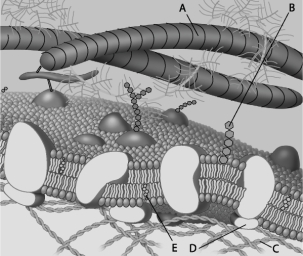 Figure 7.1
Figure 7.1
-peripheral protein
 Figure 7.1
Figure 7.1-peripheral protein

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
14
For the following questions, match the labeled component of the cell membrane (Figure 7.1) with its description.
 Figure 7.1
Figure 7.1
-cholesterol
 Figure 7.1
Figure 7.1-cholesterol

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
15
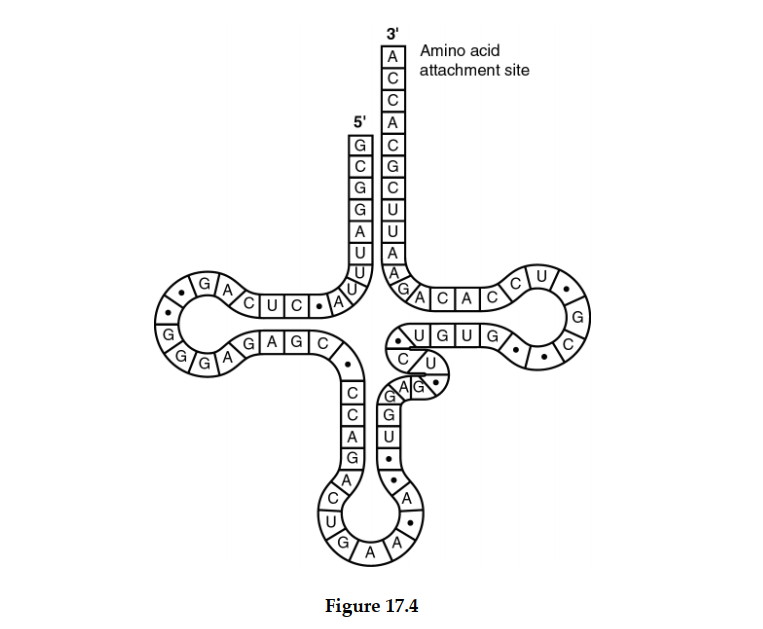
-The tRNA shown in Figure 17.4 has its 3 end projecting beyond its 5 end. What will occur at this 3 end?
A) The codon and anticodon complement one another.
B) The amino acid binds covalently.
C) The excess nucleotides (ACCA) will be cleaved off at the ribosome.
D) The small and large subunits of the ribosome will attach to it.
E) The 5? cap of the mRNA will become covalently bound.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
16
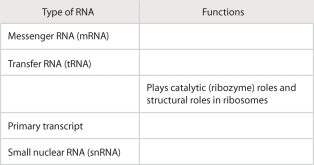
Review the roles of RNA by filling in the following table:


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
17
Exponential growth of a population is represented by dN/dt =
A)
B)rN
C)rN (K + N)
D)
E)
A)
B)rN
C)rN (K + N)
D)
E)

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
18
Logistic growth of a population is represented by dN/dt =
A)
B)rN
C)rN (K + N)
D)
E)
A)
B)rN
C)rN (K + N)
D)
E)

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following graphs refer to this equation?
A)
B)
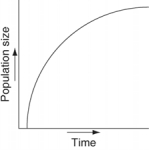
C)
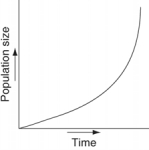
D)
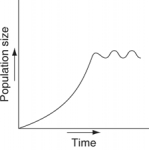
E)
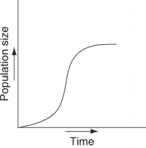
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
20
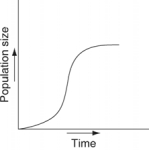
Which of the following graphs illustrates the growth curve of a small population of rodents that has grown to reach a static carrying capacity?
A)
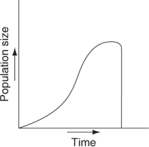
B)
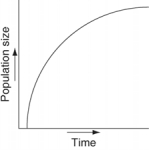
C)
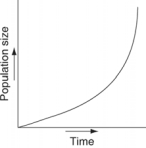
D)
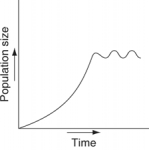
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
21
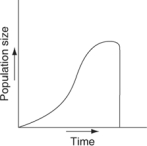
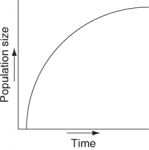
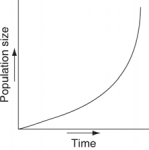
Which of the following graphs illustrates the growth curve of a population of snowshoe hares over several seasons in northern Canada?
A)
B)
C)
D)
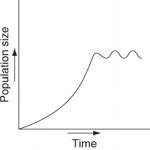
E)
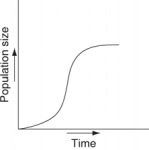
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
22
When levels of CO2 are experimentally increased, C3 plants generally respond with a greater increase in productivity than C4 plants. This is because
A) C3 plants are more efficient in their use of CO2.
B) C3 plants are able to obtain the same amount of CO 2 by keeping their stomata open for shorter periods of time.
C) C4 plants dont use CO2 as their source of carbon.
D) C3 plants are more limited than C4 plants by CO2 availability because of transpirational water loss.
E) C3 plants have special adaptations for CO2 uptake, such as larger stomata.
A) C3 plants are more efficient in their use of CO2.
B) C3 plants are able to obtain the same amount of CO 2 by keeping their stomata open for shorter periods of time.
C) C4 plants dont use CO2 as their source of carbon.
D) C3 plants are more limited than C4 plants by CO2 availability because of transpirational water loss.
E) C3 plants have special adaptations for CO2 uptake, such as larger stomata.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
23
If P = 0.3 MPa and S = -0.45 MPa, the resulting is
A) +0.75 MPa.
B) -0.75 MPa.
C) -0.15 MPa.
D) +0.15 MPa.
E) -0.42 MPa.
A) +0.75 MPa.
B) -0.75 MPa.
C) -0.15 MPa.
D) +0.15 MPa.
E) -0.42 MPa.

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following seems to be the known upper and lower size limits of genomes?
A) 1-2900 Mb (million base pairs)
B) 1,500-40,000 Mb
C) 1-580,000 Mb
D) 100-120,000 Mb
E) 100-200,000 Mb
A) 1-2900 Mb (million base pairs)
B) 1,500-40,000 Mb
C) 1-580,000 Mb
D) 100-120,000 Mb
E) 100-200,000 Mb

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck
25
The following questions refer to this information:
In the year 2500, five male space colonists and five female space colonists (all unrelated to each other) settle on an uninhabited Earthlike planet in the Andromeda galaxy. The colonists and their offspring randomly mate for generations. All ten of the original colonists had free earlobes, and two were heterozygous for that trait. The allele for free earlobes is dominant to the allele for attached earlobes
-Which of these is closest to the allele frequency in the founding population?
A) 0.1 a, 0.9 A
B) 0.2 a, 0.8 A
C) 0.5 a, 0.5 A
D) 0.8 a, 0.2 A
E) 0.4 a, 0.6 A
In the year 2500, five male space colonists and five female space colonists (all unrelated to each other) settle on an uninhabited Earthlike planet in the Andromeda galaxy. The colonists and their offspring randomly mate for generations. All ten of the original colonists had free earlobes, and two were heterozygous for that trait. The allele for free earlobes is dominant to the allele for attached earlobes
-Which of these is closest to the allele frequency in the founding population?
A) 0.1 a, 0.9 A
B) 0.2 a, 0.8 A
C) 0.5 a, 0.5 A
D) 0.8 a, 0.2 A
E) 0.4 a, 0.6 A

Unlock Deck
Unlock for access to all 25 flashcards in this deck.
Unlock Deck
k this deck


