Deck 15: Oxidation and Reduction
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/70
Play
Full screen (f)
Deck 15: Oxidation and Reduction
1
Which of the following statements is not true of a redox reaction?
A) The reducing agent is the substance oxidized.
B) The reducing agent gains electrons.
C) The substance oxidized loses electrons.
D) The oxidizing agent is the substance reduced.
A) The reducing agent is the substance oxidized.
B) The reducing agent gains electrons.
C) The substance oxidized loses electrons.
D) The oxidizing agent is the substance reduced.
The reducing agent gains electrons.
2
In the following reaction H2SO4 is ________.
H2SO4 + HI I2 + SO2 + H2O
A) the oxidizing agent and is oxidized
B) the oxidizing agent and is reduced
C) the reducing agent and is oxidized
D) the reducing agent and is reduced
H2SO4 + HI I2 + SO2 + H2O
A) the oxidizing agent and is oxidized
B) the oxidizing agent and is reduced
C) the reducing agent and is oxidized
D) the reducing agent and is reduced
the oxidizing agent and is reduced
3
Indicate the missing words (or phrases) in the following definition: "An oxidation number is the ________ that an atom ________ when the electrons in each bond it is participating in are assigned to the ________ electronegative of the two atoms involved in the bond."
A) number of electrons, definitely has, more
B) number of electrons, appears to have, less
C) charge, appears to have, more
D) charge, definitely has, less
A) number of electrons, definitely has, more
B) number of electrons, appears to have, less
C) charge, appears to have, more
D) charge, definitely has, less
charge, appears to have, more
4
The proper assignment of oxidation numbers to the elements in Na2CrO4 would be ________.
A) +1 for Na, +6 for Cr and -2 for O
B) +2 for Na, +4 for Cr and -6 for O
C) +2 for Na, +3 for Cr and -2 for O
D) +2 for Na, +5 for Cr and -6 for O
A) +1 for Na, +6 for Cr and -2 for O
B) +2 for Na, +4 for Cr and -6 for O
C) +2 for Na, +3 for Cr and -2 for O
D) +2 for Na, +5 for Cr and -6 for O

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
5
+7 is the oxidation number of ________.
A) C in MgC2O4
B) S in H2SO4
C) Mn in KMnO4
D) Br in NaBrO3
A) C in MgC2O4
B) S in H2SO4
C) Mn in KMnO4
D) Br in NaBrO3

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
6
The oxidation number of C in BaC2O4 is ________.
A) -3
B)-2
C) +2
D) +3
A) -3
B)-2
C) +2
D) +3

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
7
The oxidation number of Cr in K2Cr2O7 is ________.
A) +6
B) +5
C) +4
D) +2
A) +6
B) +5
C) +4
D) +2

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
8
In which of the following compounds does Cl have an oxidation number of +7?
A) LiClO3
B) Al(ClO4)3
C) Ca(ClO3)2
D) NaClO2
A) LiClO3
B) Al(ClO4)3
C) Ca(ClO3)2
D) NaClO2

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
9
In which of the following sequences of sulfur-containing species are the species arranged in order of decreasing oxidation number for S?
A) SO32-, SO42-, S2-
B) SO42-, S2O32-, S2-
C) S2O32-, SO32-, S2-
D) SO42-, S2-, S2O32-
A) SO32-, SO42-, S2-
B) SO42-, S2O32-, S2-
C) S2O32-, SO32-, S2-
D) SO42-, S2-, S2O32-

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
10
In which of the following compounds is the oxidation number of oxygen not ?2?
A) Ba(OH)2
B) Li2O2
C) NaClO2
D) Na2SO4
A) Ba(OH)2
B) Li2O2
C) NaClO2
D) Na2SO4

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
11
In which of the following compounds is the oxidation number of hydrogen not +1?
A) NaH
B) H2SO4
C) NaClO2
D) NH3
A) NaH
B) H2SO4
C) NaClO2
D) NH3

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
12
The pure element zinc has an oxidation number of ________, while in a compound like ZnSO4 the oxidation number for zinc is ________.
A) 0, 0
B) 0, +1
C) +1, 0
D) 0, +2
A) 0, 0
B) 0, +1
C) +1, 0
D) 0, +2

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
13
In the redox reaction: Al + MnO2 Al2O3 + Mn, the oxidizing agent is ________.
A) Al
B) Mn in MnO2
C) O in MnO2
D) O in Al2O3
A) Al
B) Mn in MnO2
C) O in MnO2
D) O in Al2O3

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
14
In a redox reaction, the substance reduced ________.
A) contains an element which increases in oxidation number
B) is also the reducing agent
C) always gains electrons
D) always loses electrons
A) contains an element which increases in oxidation number
B) is also the reducing agent
C) always gains electrons
D) always loses electrons

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
15
In the redox reaction: BaSO4 + 4 C BaS + 4 CO, the element reduced is ________.
A) S in BaSO4
B) C
C) Ba in BaS
D) O in CO
A) S in BaSO4
B) C
C) Ba in BaS
D) O in CO

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following accurately represents the oxidation of the Co+2 ion?
A) Co+2 + 2 e- Co
B) Co+2 Co+3 + e-
C) Co Co+2 + 2 e-
D) Co+3 + e- Co+2
A) Co+2 + 2 e- Co
B) Co+2 Co+3 + e-
C) Co Co+2 + 2 e-
D) Co+3 + e- Co+2

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
17
Which reaction below can be classified as a redox/decomposition reaction?
A) 2 CuO 2 Cu + O2
B) 2 NO + O2 2 NO2
C) Sn + Cu(NO3)2 Sn(NO3)2 + Cu
D) Cu(NO3)2 + 2 NaOH Cu(OH)2 + 2 NaNO3
A) 2 CuO 2 Cu + O2
B) 2 NO + O2 2 NO2
C) Sn + Cu(NO3)2 Sn(NO3)2 + Cu
D) Cu(NO3)2 + 2 NaOH Cu(OH)2 + 2 NaNO3

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following equations is incorrectly classified as to type of chemical reaction?
A) CaCO3 CaO + CO2 decomposition/redox
B) H2O + SO2 H2SO3 synthesis/nonredox
C) Cl2 + F2 2 ClF synthesis/redox
D) AgNO3 + NaCl AgCl + NaNO3 double-displacement/non-redox
A) CaCO3 CaO + CO2 decomposition/redox
B) H2O + SO2 H2SO3 synthesis/nonredox
C) Cl2 + F2 2 ClF synthesis/redox
D) AgNO3 + NaCl AgCl + NaNO3 double-displacement/non-redox

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following equations is incorrectly classified as to type of chemical reaction?
A) NaHCO3 + HCl NaCl + H2O + CO2 double-replacement/nonredox
B) 2 Na + H2 2 NaH synthesis/redox
C) PbO + C Pb + CO single-replacement/nonredox
D) 2 Na + 2 HCl 2 NaCl + H2 single-replacement/redox
A) NaHCO3 + HCl NaCl + H2O + CO2 double-replacement/nonredox
B) 2 Na + H2 2 NaH synthesis/redox
C) PbO + C Pb + CO single-replacement/nonredox
D) 2 Na + 2 HCl 2 NaCl + H2 single-replacement/redox

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
20
Determine the oxidation number of C in NaHCO3.
A) +6
B) +12
C) +4
D) +5
A) +6
B) +12
C) +4
D) +5

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
21
Determine the oxidation number of the underlined element in NaBrO4.
A) +7
B) +4
C) -6
D) -4
A) +7
B) +4
C) -6
D) -4

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
22
In which of the following compounds does nitrogen have an oxidation number of +4?
A) HNO3
B) NO2
C) N2O
D) NH4Cl
A) HNO3
B) NO2
C) N2O
D) NH4Cl

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
23
The following reactions are classified first as redox or nonredox. They are further classified as synthesis, decomposition, single replacement, or double displacement reactions. Which of the following reactions is incorrectly classified as to redox/nonredox or as to synthesis, decomposition, single-replacement, or double-displacement (precipitation) reactions?
A) HNO3(aq) + LiOH(aq) LiNO3 (aq) + H2O(l) non-redox / double-displacement
B) Pb(NO3)2(aq) + 2 Na(s) Pb(s) + NaNO3(aq) redox / single-replacement
C) 2 H2O2(l) 2 H2O(l) + O2(g) non-redox / decomposition
D) AgNO3(aq) + KOH(aq) KNO3(aq) + AgOH(s) non-redox / precipitation
A) HNO3(aq) + LiOH(aq) LiNO3 (aq) + H2O(l) non-redox / double-displacement
B) Pb(NO3)2(aq) + 2 Na(s) Pb(s) + NaNO3(aq) redox / single-replacement
C) 2 H2O2(l) 2 H2O(l) + O2(g) non-redox / decomposition
D) AgNO3(aq) + KOH(aq) KNO3(aq) + AgOH(s) non-redox / precipitation

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following reactions is a redox single replacement reaction?
A) Zn + H2SO4 H2 + ZnSO4
B) 2 KClO3 2 KCl + O2
C) 2 NO2 + H2O2 2 HNO3
D) SO3 + H2O H2SO4
A) Zn + H2SO4 H2 + ZnSO4
B) 2 KClO3 2 KCl + O2
C) 2 NO2 + H2O2 2 HNO3
D) SO3 + H2O H2SO4

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following reactions is not a redox reaction?
A) SO3 + H2O H2SO4
B) 2 Na + Mg2+ Mg + 2 Na+
C) Cl2 + 2I- 2 Cl- + I2
D) Na + AgNO3 NaNO3 + Ag
A) SO3 + H2O H2SO4
B) 2 Na + Mg2+ Mg + 2 Na+
C) Cl2 + 2I- 2 Cl- + I2
D) Na + AgNO3 NaNO3 + Ag

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
26
In the redox reaction: 4 Cr + 3 O2 + 12 HBr 4 CrBr3 + 6 H2O the change in oxidation number for O is from ________.
A) +4 to -4
B) +2 to 0
C) 0 to -2
D) +3 to 0
A) +4 to -4
B) +2 to 0
C) 0 to -2
D) +3 to 0

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
27
In the redox reaction: 2 HNO2 + 2 HI 2 NO + I2 + 2 H2O, ________.
A) HNO2 is the oxidizing agent.
B) HNO2 is the substance oxidized.
C) I undergoes an oxidation number increase of 2 units per atom.
D) N undergoes an oxidation number increase of 2 units per atom.
A) HNO2 is the oxidizing agent.
B) HNO2 is the substance oxidized.
C) I undergoes an oxidation number increase of 2 units per atom.
D) N undergoes an oxidation number increase of 2 units per atom.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
28
In the following reaction: 2 Mn+2 + Br2 2 Mn+3 + 2 Br- the species that is oxidized is ________.
A) Mn2+
B) Br-
C) Mn+3
D) Br2
A) Mn2+
B) Br-
C) Mn+3
D) Br2

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
29
In the following reaction, which species is the reducing agent?
3 Cu (s) + 6 H+ (aq) + 2 HNO3 (aq) 3 Cu+2 (aq) + 2 NO (g) + 4 H2O (l)
A) H+
B) Cu
C) N in NO
D) Cu+2
3 Cu (s) + 6 H+ (aq) + 2 HNO3 (aq) 3 Cu+2 (aq) + 2 NO (g) + 4 H2O (l)
A) H+
B) Cu
C) N in NO
D) Cu+2

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
30
Which substance is serving as the reducing agent in the following reaction?
Fe2S3 + 12 HNO3 2 Fe(NO3)3 + 3 S + 6 NO2 + 6 H2O
A) HNO3
B) S
C) NO2
D) Fe2S3
Fe2S3 + 12 HNO3 2 Fe(NO3)3 + 3 S + 6 NO2 + 6 H2O
A) HNO3
B) S
C) NO2
D) Fe2S3

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
31
In the following reaction: 2 Al (s) + 3 I2 (s) 2 AlI3 (s) which species is the oxidizing agent?
A) Al
B) AlI3
C) I2
D) none of the species
A) Al
B) AlI3
C) I2
D) none of the species

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
32
Which substance is serving as the reducing agent in the following reaction?
14H+ + Cr2O7-2 + 3 Ni 3 Ni2+ + 2 Cr+3 + 7 H2O
A) Ni
B) H+
C) Cr2O7-2
D) H2O
14H+ + Cr2O7-2 + 3 Ni 3 Ni2+ + 2 Cr+3 + 7 H2O
A) Ni
B) H+
C) Cr2O7-2
D) H2O

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
33
Which substance is serving as the oxidizing agent in the following reaction?
14H+ + Cr2O7-2 + 3 Ni 3 Ni2+ + 2 Cr+3 + 7 H2O
A) Ni
B) H+
C) Cr2O7-2
D) H2O
14H+ + Cr2O7-2 + 3 Ni 3 Ni2+ + 2 Cr+3 + 7 H2O
A) Ni
B) H+
C) Cr2O7-2
D) H2O

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
34
How many electrons are lost or gained by each formula unit of CuBr2 in the reaction
Zn + CuBr2 ZnBr2 + Cu
A) gains 2 electrons
B) loses 2 electrons
C) loses 1 electron
D) gains 6 electrons
Zn + CuBr2 ZnBr2 + Cu
A) gains 2 electrons
B) loses 2 electrons
C) loses 1 electron
D) gains 6 electrons

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is a correctly balanced oxidation half-reaction?
A) H2C2O4 2 CO2 + 2 H+ + 2e?
B) I2 + 2e- 2 I-
C) HOCl + H+ + 2e- Cl- + H2O
D) H2S S + 2 H+ + 4e-
A) H2C2O4 2 CO2 + 2 H+ + 2e?
B) I2 + 2e- 2 I-
C) HOCl + H+ + 2e- Cl- + H2O
D) H2S S + 2 H+ + 4e-

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
36
The balanced full-equation obtained by adding the following two balanced half-reactions is ________.
2H2O + PH3 H3PO2 + 4H+ + 4e-
I2 + 2e- 2I-
A) 2 H2O + PH3 + I2 H3PO2 + 4 H+ + 2 I- + 2 e-
B) 4 H2O + 2 PH3 + I2 2 H3PO2 + 8 H+ + 2 I-
C) 2 H2O + PH3 + 2 I2 H3PO2 + 8 I- + 8 H+
D) 2 H2O + PH3 + 2 I2 H3PO2 + 4 I- + 4 H+
2H2O + PH3 H3PO2 + 4H+ + 4e-
I2 + 2e- 2I-
A) 2 H2O + PH3 + I2 H3PO2 + 4 H+ + 2 I- + 2 e-
B) 4 H2O + 2 PH3 + I2 2 H3PO2 + 8 H+ + 2 I-
C) 2 H2O + PH3 + 2 I2 H3PO2 + 8 I- + 8 H+
D) 2 H2O + PH3 + 2 I2 H3PO2 + 4 I- + 4 H+

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
37
When the half-reaction: MnO4- Mn2+ (acidic solution) is correctly balanced, ________.
A) the H+ and H2O are both on the left side of the equation
B) the H+ and H2O are both on the right side of the equation
C) the H+ is on the left side, and the H2O on the right side of the equation
D) the H+ is on the right side, and the H2O on the left side of the equation
A) the H+ and H2O are both on the left side of the equation
B) the H+ and H2O are both on the right side of the equation
C) the H+ is on the left side, and the H2O on the right side of the equation
D) the H+ is on the right side, and the H2O on the left side of the equation

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
38
When the redox reaction: NF3 + AlCl3 N2 + Cl2 + AlF3 is balanced, the correct coefficients for NF3 and Cl2, respectively, are ________.
A) 3 and 4
B) 4 and 2
C) 2 and 3
D) 6 and 3
A) 3 and 4
B) 4 and 2
C) 2 and 3
D) 6 and 3

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
39
When the redox reaction: Zn + As2O3 AsH3 + Zn2+ (acidic solution) is balanced, the correct coefficients for H2O, H+ and AsH3 are, respectively, ________.
A) 6, 6 and 3
B) 6, 3 and 12
C) 3, 12 and 2
D) 4, 8 and 3
A) 6, 6 and 3
B) 6, 3 and 12
C) 3, 12 and 2
D) 4, 8 and 3

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
40
When the following equation is balanced, by the half reaction method, in an acidic solution what is the sum of the coefficients?
HNO2 + Cr2O7-2 Cr+3 + NO3-
A) 17
B) 18
C) 19
D) 20
HNO2 + Cr2O7-2 Cr+3 + NO3-
A) 17
B) 18
C) 19
D) 20

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
41
Which is the balanced half reaction in basic solution for the reaction below?
Cr(OH)4- CrO42-
A) 3 e- + Cr(OH)4- + 2 OH- Cr(OH)4- + 2 H2O
B) 4 OH- + Cr(OH)4- CrO42- + 3e- + 4 H2O
C) 2 OH- + Cr(OH)4- CrO42- + 6 e- + 3 H2O
D) Cr(OH)4- CrO42- + 4 H2O + 2 e-
Cr(OH)4- CrO42-
A) 3 e- + Cr(OH)4- + 2 OH- Cr(OH)4- + 2 H2O
B) 4 OH- + Cr(OH)4- CrO42- + 3e- + 4 H2O
C) 2 OH- + Cr(OH)4- CrO42- + 6 e- + 3 H2O
D) Cr(OH)4- CrO42- + 4 H2O + 2 e-

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
42
Which reaction is the correctly balanced half reaction (in acid solution) for the process below?
Cr2O72- (aq) Cr3+ (aq)
A) 14 H+ + Cr2O72- + 6 e- 2 Cr3+ + 7 H2O
B) 8 H+ + Cr2O7 + 3 e- 2Cr3+ + 4 H2O
C) 8 H+ + Cr2O7 2 Cr3+ + 4 H2O + 3 e-
D) 12 H+ + Cr2O72- + 3 e- 2 Cr3+ + 6 H2O
Cr2O72- (aq) Cr3+ (aq)
A) 14 H+ + Cr2O72- + 6 e- 2 Cr3+ + 7 H2O
B) 8 H+ + Cr2O7 + 3 e- 2Cr3+ + 4 H2O
C) 8 H+ + Cr2O7 2 Cr3+ + 4 H2O + 3 e-
D) 12 H+ + Cr2O72- + 3 e- 2 Cr3+ + 6 H2O

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
43
Which response represents the balanced half reaction for oxidation for the reaction given below?
HCl (aq) + Fe (s) FeCl3 (aq) + H2 (g)
A) 6 e- + 6 H+ 3 H2
B) 2 Fe 2 Fe3+ + 6 e-
C) 3 Fe Fe3+ + 3 e-
D) Fe + 3 e- Fe3+
HCl (aq) + Fe (s) FeCl3 (aq) + H2 (g)
A) 6 e- + 6 H+ 3 H2
B) 2 Fe 2 Fe3+ + 6 e-
C) 3 Fe Fe3+ + 3 e-
D) Fe + 3 e- Fe3+

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
44
Which response represents the balanced half reaction for reduction for the reaction given below?
Fe (s) + CuSO4 (aq) Fe2(SO4)3 (aq) + Cu (s)
A) 2 Cu2+ + 3 e- 2 Cu
B) Fe + 3 e- Fe3+
C) 3 Cu2+ + 6 e- 3 Cu
D) 2 Fe 2 Fe3+ + 6e-
Fe (s) + CuSO4 (aq) Fe2(SO4)3 (aq) + Cu (s)
A) 2 Cu2+ + 3 e- 2 Cu
B) Fe + 3 e- Fe3+
C) 3 Cu2+ + 6 e- 3 Cu
D) 2 Fe 2 Fe3+ + 6e-

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
45
Balance the following net ionic equation using the half reaction method (in acidic solution). What is the coefficient for MnO4-?
H+ + Fe2+ + MnO4- Fe3+ + Mn2+ + H2O
A) 8
B) 5
C) 1
D) 14
H+ + Fe2+ + MnO4- Fe3+ + Mn2+ + H2O
A) 8
B) 5
C) 1
D) 14

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
46
Balance the following net ionic equation using the half reaction method (in acidic solution). What is the sum of the coefficients for the balanced reaction?
MnO4- + SO32- Mn2+ + SO42-
A) 27
B) 29
C) 21
D) 23
MnO4- + SO32- Mn2+ + SO42-
A) 27
B) 29
C) 21
D) 23

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following reactions is a disproportionation reaction?
A) 2 H2O 2 H2 + O2
B) H2SO3 H2O + SO2
C) HNO2 NO + NO3-
D) Mg + H2SO4 MgSO4 + H2
A) 2 H2O 2 H2 + O2
B) H2SO3 H2O + SO2
C) HNO2 NO + NO3-
D) Mg + H2SO4 MgSO4 + H2

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
48
In balancing the equation for a disproportionation reaction, using the oxidation number method, the substance undergoing disproportionation is ________.
A) initially written twice on the reactant side of the equation
B) initially written twice on the product side of the equation
C) initially written on both the reactant and product sides of the equation
D) always assigned an oxidation number of zero
A) initially written twice on the reactant side of the equation
B) initially written twice on the product side of the equation
C) initially written on both the reactant and product sides of the equation
D) always assigned an oxidation number of zero

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
49
In the disproportion equation below, what species is undergoing disproportionation?
3 Br2 + 6 OH- BrO3- + 5 Br- + 3 H2O
A) OH-
B) Br-
C) H2O
D) Br2
3 Br2 + 6 OH- BrO3- + 5 Br- + 3 H2O
A) OH-
B) Br-
C) H2O
D) Br2

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following formula unit or net ionic equations represents a disproportionation reaction?
A) Fe + 3 Ag+ Fe3+ + 3 Ag
B) 2 HNO3 + SO2 H2SO4 + 2 NO2
C) ClO- + Cl- + 2 H+ Cl2 + H2O
D) CH4 + 2 O2 CO2 + H2O
A) Fe + 3 Ag+ Fe3+ + 3 Ag
B) 2 HNO3 + SO2 H2SO4 + 2 NO2
C) ClO- + Cl- + 2 H+ Cl2 + H2O
D) CH4 + 2 O2 CO2 + H2O

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following is a correct statement about galvanic cells?
A) Chemical energy is converted entirely into heat energy.
B) They are always based on a nonspontaneous redox reaction.
C) They may employ an oxidation process or a reduction process but never both.
D) Electrical energy is produced from a spontaneous redox reaction.
A) Chemical energy is converted entirely into heat energy.
B) They are always based on a nonspontaneous redox reaction.
C) They may employ an oxidation process or a reduction process but never both.
D) Electrical energy is produced from a spontaneous redox reaction.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
52
The anode in a galvanic or electrolysis cell is ________.
A) the compartment where solution is placed
B) the electrode where electrons leave the cell
C) the electrode where reduction takes place
D) always the oxidizing agent for the reaction
A) the compartment where solution is placed
B) the electrode where electrons leave the cell
C) the electrode where reduction takes place
D) always the oxidizing agent for the reaction

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
53
In a galvanic cell, the cathode is the electrode at which ________ occurs?
A) oxidation
B) reduction
C) oxidation and reduction
D) nothing
A) oxidation
B) reduction
C) oxidation and reduction
D) nothing

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following is not correct for both acidic and alkaline dry cells?
A) As the name implies, the electrolyte is a dry material.
B) The anode reaction involves Zn.
C) The cathode reaction involves MnO2.
D) The cathode is an inert material.
A) As the name implies, the electrolyte is a dry material.
B) The anode reaction involves Zn.
C) The cathode reaction involves MnO2.
D) The cathode is an inert material.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following statements concerning the lead storage battery is incorrect?
A) PbSO4 is produced during the discharging of the battery.
B) PbO2 is consumed during the recharging of the battery.
C) Pb serves as the anode.
D) Pb coated with PbO2 serves as the cathode.
A) PbSO4 is produced during the discharging of the battery.
B) PbO2 is consumed during the recharging of the battery.
C) Pb serves as the anode.
D) Pb coated with PbO2 serves as the cathode.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
56
During the recharging of a lead storage battery, what products(s) are formed?
A) PbO2 (s) and PbSO4 (s)
B) Pb (s) and PbO2 (s)
C) Pb (s) only
D) PbO2 (s) only
A) PbO2 (s) and PbSO4 (s)
B) Pb (s) and PbO2 (s)
C) Pb (s) only
D) PbO2 (s) only

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
57
Which statement is incorrect?
A) Reduction occurs at the anode in an electrolytic cell.
B) Electroplating is the deposition of a thin layer of metal on an object.
C) During recharging the lead storage battery is functioning as an electrolytic cell.
D) Electrolysis is a process in which electrical energy is used to cause a nonspontaneous reaction to occur.
A) Reduction occurs at the anode in an electrolytic cell.
B) Electroplating is the deposition of a thin layer of metal on an object.
C) During recharging the lead storage battery is functioning as an electrolytic cell.
D) Electrolysis is a process in which electrical energy is used to cause a nonspontaneous reaction to occur.

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
58
In the reactions below, which reaction correctly identifies the anode reaction during the discharging (normal usage) of a lead storage battery cell?
A) Pb + SO42- PbSO4 + 2 e-
B) Zn + 2 OH- ZnO + H2O + 2 e-
C) 2 MnO2 + H2O + 2 e- Mn2O3 + 2 OH-
D) PbO2 + 4 H+ + SO42- + 2 e- PbSO4 + 2 H2O
A) Pb + SO42- PbSO4 + 2 e-
B) Zn + 2 OH- ZnO + H2O + 2 e-
C) 2 MnO2 + H2O + 2 e- Mn2O3 + 2 OH-
D) PbO2 + 4 H+ + SO42- + 2 e- PbSO4 + 2 H2O

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
59
What is the cathode material in a common dry cell such as a flashlight battery?
A) lead
B) graphite
C) zinc
D) magnesium
A) lead
B) graphite
C) zinc
D) magnesium

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
60
Assign an oxidation number to each atom in the following chemical reaction.
As2O3 + 5 H2O + 2 I2 ? 2 H3AsO4 + 4 HI
As2O3 + 5 H2O + 2 I2 ? 2 H3AsO4 + 4 HI

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
61
Identify which substance is oxidized and which substance is reduced in the following chemical reaction.
4 NH3 + 3 O2 → 2 N2 + 6 H2O
4 NH3 + 3 O2 → 2 N2 + 6 H2O

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
62
Indicate whether each of the following formula unit or net ionic equations is or is not a redox reaction by writing the word
-NH4+(aq) + CN-(aq) HCN(aq) + NH3(aq) ____________________
A) non-redox
B) redox
-NH4+(aq) + CN-(aq) HCN(aq) + NH3(aq) ____________________
A) non-redox
B) redox

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
63
Indicate whether each of the following formula unit or net ionic equations is or is not a redox reaction by writing the word
-2 Al(s) + 3 FeO(s) 3 Fe(s) + Al2O3(s) ____________________
A) non-redox
B) redox
-2 Al(s) + 3 FeO(s) 3 Fe(s) + Al2O3(s) ____________________
A) non-redox
B) redox

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
64
Indicate whether each of the following formula unit or net ionic equations is or is not a redox reaction by writing the word
-N2(g) + 3 H2(g) 2 NH3(g) ____________________
A) non-redox
B) redox
-N2(g) + 3 H2(g) 2 NH3(g) ____________________
A) non-redox
B) redox

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
65
Indicate whether each of the following formula unit or net ionic equations is or is not a redox reaction by writing the word
-Ag+(aq) + Cl-(aq) AgCl(s) ____________________
A) non-redox
B) redox
-Ag+(aq) + Cl-(aq) AgCl(s) ____________________
A) non-redox
B) redox

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
66
Balance the following equation by the oxidation-number method.
Fe2O3 + CO → Fe + CO2
Fe2O3 + CO → Fe + CO2

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
67
Balance the following half reaction in basic solution.
S2? ? SO32?
S2? ? SO32?

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
68
Balance the following half reaction in acidic solution.
NO3? ? NO
NO3? ? NO

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
69
Write the balanced half reactions for oxidation and reduction for the following chemical equation.
Fe (s) + Cu(NO3)2 (aq) → Fe(NO3)3 (aq) + Cu (s)
Fe (s) + Cu(NO3)2 (aq) → Fe(NO3)3 (aq) + Cu (s)

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck
70
Balance the following reactions for the solution type indicted. Be sure to identify which is oxidized and reduced.
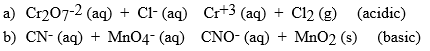
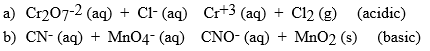

Unlock Deck
Unlock for access to all 70 flashcards in this deck.
Unlock Deck
k this deck


