Deck 2: Crystallization
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/11
Play
Full screen (f)
Deck 2: Crystallization
1
Each of the following compounds, A-C, is equally soluble in the three solvents listed. In each case, which solvent would you choose? Give reasons for your answer. (More than one answer
may be correct.)
(a) Compound A: benzene, acetone, or chloroform
(b) Compound B: chloroform, methylene chloride, or ethyl acetate
(c) Compound C: methanol, ethanol, or water
may be correct.)
(a) Compound A: benzene, acetone, or chloroform
(b) Compound B: chloroform, methylene chloride, or ethyl acetate
(c) Compound C: methanol, ethanol, or water
(a) Acetone is the solvent of choice because it has the lowest boiling pint and is the least toxic of the three.
(b) Ethyl acetate is the solvent of choice because it is less toxic than chloroform or methylene chloride. Ethyl acetate is sufficiently volatile that it can be removed easily from the sample.
(c) Any of these would be a reasonable choice, but ethanol would probably be the best. It is less toxic than methanol. Although ethanol is flammable, it is more vlatile than water and would be removed easily from the crystals.
(b) Ethyl acetate is the solvent of choice because it is less toxic than chloroform or methylene chloride. Ethyl acetate is sufficiently volatile that it can be removed easily from the sample.
(c) Any of these would be a reasonable choice, but ethanol would probably be the best. It is less toxic than methanol. Although ethanol is flammable, it is more vlatile than water and would be removed easily from the crystals.
2
Which of the following solvents could not be used as solvent pairs for crystallization? Explain. (Hint: A solvent pair must be two miscible liquids; see Table 3.1, p. 51.)
(a) Hexanes and water
(b) Chloroform and diethyl ether
(c) Acetone and methanol
(a) Hexanes and water
(b) Chloroform and diethyl ether
(c) Acetone and methanol
(a) yes; this solvent pair is immiscible.
(b) no; this solvent pair is miscible
(c) no; this solvent pair is miscible
(b) no; this solvent pair is miscible
(c) no; this solvent pair is miscible
3
3 . Suggest possible crystallization solvents for the following compounds.
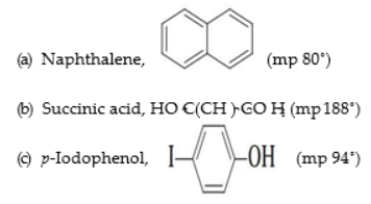

.
(a) Naphthalene is an aromatic hydrocarbon. Therefore, benzene would be a logical choice. However, benzene is quite toxic and should be avoided whenever possible. Hexanes or petroleum ether would probably be a good choice.
(b) Succinic acid is a polar, hydrogen-bonding compound. you would expect it to be soluble in water and insoluble in nonpolar solvents such as petroleum ether. Therefore, a solvent of moderate polarity such as acetone, ethyl acetate, or ethanol would be a good choice.
(c) p-Iodophenol contains a nonpolar iodo group and a hydrogen-bonding hydroxyl group. Therefore, water or water-ethanol would be a good choice.
(a) Naphthalene is an aromatic hydrocarbon. Therefore, benzene would be a logical choice. However, benzene is quite toxic and should be avoided whenever possible. Hexanes or petroleum ether would probably be a good choice.
(b) Succinic acid is a polar, hydrogen-bonding compound. you would expect it to be soluble in water and insoluble in nonpolar solvents such as petroleum ether. Therefore, a solvent of moderate polarity such as acetone, ethyl acetate, or ethanol would be a good choice.
(c) p-Iodophenol contains a nonpolar iodo group and a hydrogen-bonding hydroxyl group. Therefore, water or water-ethanol would be a good choice.
4
Give reasons for each of the following experimental techniques used in crystallization.
(a) A hot crystallization solution is not filtered unless absolutely necessary.
(b) An Erlenmeyer flask containing a hot solution is not tightly stoppered to prevent solvent loss during cooling.
(c) The suction of a vacuum filtration apparatus is broken before the vacuum is turned off.
(d) Vacuum filtration is avoided when crystals are isolated from a very volatile solvent.
(a) A hot crystallization solution is not filtered unless absolutely necessary.
(b) An Erlenmeyer flask containing a hot solution is not tightly stoppered to prevent solvent loss during cooling.
(c) The suction of a vacuum filtration apparatus is broken before the vacuum is turned off.
(d) Vacuum filtration is avoided when crystals are isolated from a very volatile solvent.

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
5
A student was recrystallizing a compound. As the hot solution cooled to room temperature, no crystals appeared. The flask was then placed in an ice-water bath. Suddenly a large amount of solid material appeared in the flask. The student isolated a good yield of product; however, the product was contaminated with impurities. Explain.

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
6
A chemist crystallizes 17.5 g of a solid and isolates 10.2 g as the first crop and 3.2 g as the sec- ond crop.
(a) What is the percent recovery in the first crop?
(b) What is the total percent recovery?
(a) What is the percent recovery in the first crop?
(b) What is the total percent recovery?

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
7
A student crystallized a compound from benzene and observed only a few crystals when the solution cooled to room temperature. To increase the yield of crystals, the student chilled the mixture in an ice-water bath. The chilling greatly increased the quantity of solid material in the flask. Yet when the student filtered these crystals with vacuum, only a few crystals remained on the filter paper. Explain this student's observations.

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
8
The solubility of acetanilide in hot water (5.5 g / 100 mL at 100˚) is not very great, and its solu- bility in cold water (0.53 g / 100 mL at 0˚) is significant. What would be the maximum theoreti- cal percent recovery (first crop only) from the crystallization of 5.0 g of acetanilide from 100 mL of water (assuming the solution is chilled to 0˚)?

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
9
A 1.0 g sample of benzoic acid is contaminated with 0.05 g of salicylic acid. Solubilities in water of the two compounds are given in the following table.
 (a) What volume of boiling water is needed to dissolve the 1.0 g of benzoic acid?
(a) What volume of boiling water is needed to dissolve the 1.0 g of benzoic acid?
(b) How much benzoic acid will crystallize after cooling to 20?C?
(c) Will any salicylic acid crystals also form?
(d) Will the benzoic acid be pure?
 (a) What volume of boiling water is needed to dissolve the 1.0 g of benzoic acid?
(a) What volume of boiling water is needed to dissolve the 1.0 g of benzoic acid?(b) How much benzoic acid will crystallize after cooling to 20?C?
(c) Will any salicylic acid crystals also form?
(d) Will the benzoic acid be pure?

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
10
Consider a sample of 1.0 g of benzoic acid contaminated with 1.0 g of salicylic acid.
(a) What volume of boiling water irs needed to dissolve the 1.0 g of benzoic acid?
(b) How much benzoic acid will crystallize after cooling to 20˚C?
(c) Will any salicylic acid crystals also form?
(d) Will the benzoic acid be pure?
(a) What volume of boiling water irs needed to dissolve the 1.0 g of benzoic acid?
(b) How much benzoic acid will crystallize after cooling to 20˚C?
(c) Will any salicylic acid crystals also form?
(d) Will the benzoic acid be pure?

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck
11
During a crystallization, while heating a solution of a compound to dissolve it in hot solvent, you boil it so long that a substantial amount of the solvent evaporates. What is likely to hap- pen to some of the solute? What should you do if this occurs?

Unlock Deck
Unlock for access to all 11 flashcards in this deck.
Unlock Deck
k this deck


