Deck 3: Melting Points
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/10
Play
Full screen (f)
Deck 3: Melting Points
1
Look up the melting points of the following compounds in a suitable handbook, Internet site, or online source. (See Technique 17, p. 198.) Include the name of your source with your answer.
(a) Quinine
(b) Indophenol
(a) Quinine
(b) Indophenol
(a) 177 (dec) (www.chemfinder.com)
(b) 160˚ (CRC, 70 th edition )
(b) 160˚ (CRC, 70 th edition )
2
What setting should you have on the Mel-Temp apparatus when you are in the vicinity of the melting point for each of the compounds in Problem 2.1?
(a) about 4.4
(b) about 4.1
(b) about 4.1
3
What setting should you have on the Fisher-Johns apparatus when you are in the vicinity of the melting point for each of the compounds in Problem 2.1?
(a) about 70
(b) about 62
(a) about 70
(b) about 62
The Fisher-Johns apparatus should be set close to the expected melting point for each compound. Therefore, for a compound with a melting point of about 70, the apparatus should be set slightly below, around 65 to allow for a gradual increase in temperature. Similarly, for a compound with a melting point of about 62, the apparatus should be set slightly below, around 57. This allows for accurate determination of the melting point without rapidly surpassing it.
4
Using the following table, draw a melting-point diagram.
 (a) Estimate the eutectic temperature and composition.
(a) Estimate the eutectic temperature and composition.
(b) If you wished to obtain a more accurate graph for this melting-point curve, suggest the best mixtures for additional mixed melting points.
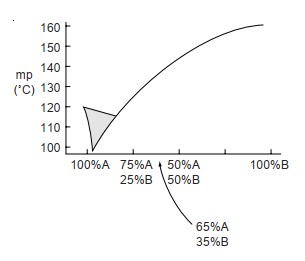
 (a) Estimate the eutectic temperature and composition.
(a) Estimate the eutectic temperature and composition.(b) If you wished to obtain a more accurate graph for this melting-point curve, suggest the best mixtures for additional mixed melting points.


Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
5
Why is it not good practice to mix two components by simply stirring them together and then sampling them for a mixed melting point?

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
6
You think that you have isolated aspirin (acetylsalicylic acid) in the lab. It melts at 75˚-77˚C. Since you don't totally trust your own laboratory techniques, you want to prove to yourself that you have aspirin. Using only melting-point techniques, explain how you can prove that you actually have aspirin. (Assume the stockroom is able to supply you with any compound you need.)

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
7
The following table lists the observed melting points for several compounds isolated in the student laboratory.
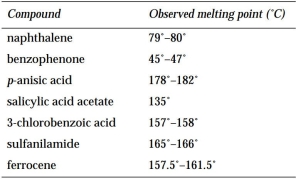 (a) Look up the melting point for each compound in a suitable resource.
(a) Look up the melting point for each compound in a suitable resource.
(b) Which compounds are pure? Which are impure?
 (a) Look up the melting point for each compound in a suitable resource.
(a) Look up the melting point for each compound in a suitable resource.(b) Which compounds are pure? Which are impure?

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
8
Suppose that you are taking a melting point and the compound disappears: What happened? Why did it do this? What should you do?

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
9
You and your lab partner take melting points of the same sample. You observe a melting point of 101˚-107˚C, while your partner observes a value of 110˚-112˚C. Explain how two different melting point values can be observed for the exact same sample.

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck
10
Using Table 2.1, construct a thermometer calibration graph from the following observed melt- ing points.
Acetanilide, mp 113˚
Benzamide, mp 130˚
Succinic acid, mp 188˚
p- Nitrobenzoic acid, mp 245˚
Acetanilide, mp 113˚
Benzamide, mp 130˚
Succinic acid, mp 188˚
p- Nitrobenzoic acid, mp 245˚

Unlock Deck
Unlock for access to all 10 flashcards in this deck.
Unlock Deck
k this deck


