Deck 7: Fractional Distillation
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/8
Play
Full screen (f)
Deck 7: Fractional Distillation
1
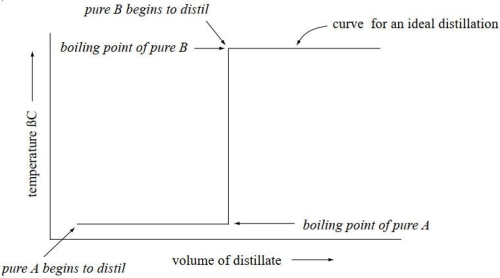
Draw an ideal distillation curve (temperature vs. milliliters of distillate) for a 50:50 mixture of two miscible liquids. How might one achieve such an ideal distillation?
An ideal distillation such as illustrated below is achieved when a fractional distillation is per- formed, using a fractionating column of the proper HETP for the boiling point difference of the two miscible liquids.

2
Why is a fractionation column packed with glass helices more efficient than a Vigreux column of the same length and same diameter?
The larger surface area of the helices permits more condensation-vaporization steps, thus increasing thnumber of theoretical plates.
3
Which of the following circumstances might contribute to column flooding and why?
(a) holes in column packing
(b) packing too tight
(c) heating too rapidly
(d) column too cold
(a) holes in column packing
(b) packing too tight
(c) heating too rapidly
(d) column too cold
Any one of the listed conditions could lead to flooding:
(a) because vapor can build up excessively, then condense
(b) because the column may be partially plugged (not allowing vapors to ascend freely) adn because the increased surface area of teh packing allows excellive condensation
(c) and (d) because excessive condensation in the column will occur
(a) because vapor can build up excessively, then condense
(b) because the column may be partially plugged (not allowing vapors to ascend freely) adn because the increased surface area of teh packing allows excellive condensation
(c) and (d) because excessive condensation in the column will occur
4
Explain why flooding in the fractionation column can lead to a poor separation of distilling components.

Unlock Deck
Unlock for access to all 8 flashcards in this deck.
Unlock Deck
k this deck
5
Referring to Tables 6.1 and 6.2, determine the approximate height of Vigreux columns needed to separate binary mixtures of compounds with the following boiling-point differences.
(a) 50˚
(b) 25˚
(c) 5˚
(a) 50˚
(b) 25˚
(c) 5˚

Unlock Deck
Unlock for access to all 8 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following pairs of compounds be separated by simple distillation? Which would require fractional distillation? (Boiling points for organic compounds are found in both online databases and printed handbooks. See Technique 17, p. 198.)
(a) n-butyl acetate and 2-ethyl-1-hexanol
(b) 3,3-dimethyl-2-butanone and 1-chloro-2-propanone
(c) cyclopentanol and 2-methyl-1-propanol
(d) 2-heptanone and n-pentyl propionate
(a) n-butyl acetate and 2-ethyl-1-hexanol
(b) 3,3-dimethyl-2-butanone and 1-chloro-2-propanone
(c) cyclopentanol and 2-methyl-1-propanol
(d) 2-heptanone and n-pentyl propionate

Unlock Deck
Unlock for access to all 8 flashcards in this deck.
Unlock Deck
k this deck
7
For each pair of compounds that require fractional distillation for separation in Problem 6, how many theoretical plates would be required? What type of fractionating column would give the proper number of theoretical plates?

Unlock Deck
Unlock for access to all 8 flashcards in this deck.
Unlock Deck
k this deck
8
A chemist has a small amount of a compound (bp 65˚) that must be fractionally distilled, yet the chemist does not want to lose any of the compound to holdup on the column. What can the chemist do?

Unlock Deck
Unlock for access to all 8 flashcards in this deck.
Unlock Deck
k this deck


