Deck 12: Column Chromatography
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/7
Play
Full screen (f)
Deck 12: Column Chromatography
1
A chemist wishes to carry out a gravity chromatographic separation using diethyl ether and ethanol as the eluting solvents.
(a) With which solvent should the chemist begin the elution?
(b) What would happen if the chemist started with the other solvent?
(a) With which solvent should the chemist begin the elution?
(b) What would happen if the chemist started with the other solvent?
(a) An elution chromatography is always started with the less polar solvent-diethyl ether, in this case.
(b) The more polsr solvent (ethanol) will carry all components through the column with little or no separation.
(b) The more polsr solvent (ethanol) will carry all components through the column with little or no separation.
2
A highly polar compound is moving through a gravity chromatography column too slowly. What can the chromatographer do to increase its rate of movement?
The chromatographer should gradually increase the polarity of the solvent mixture by adding a small amount of a more polar solvent.
3
List the following compounds in order of expected elution from a chromatography column packed with silica gel, using a petroleum ether-diethyl ether solvent system.
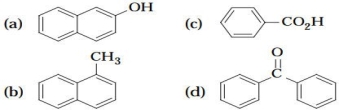

From first to last: (b) an aromatic hydrocarbon, (d) a ketone, (a) a phenol, (c) a carboxylic acid. In practice, (a) and (c) would both elute very slowly and would probably be difficult to sepa- rate.
4
What will be the result of the following errors in a gravity chromatography process?
(a) Holes or air bubbles are present in the column packing.
(b) The column is loaded with too much sample.
(c) The column is eluted first with 5% diethyl ether in hexanes, then the eluting solvent is immediately switched to 50% diethyl ether in hexanes.
(d) The column is allowed to go dry before it is completely developed.
(a) Holes or air bubbles are present in the column packing.
(b) The column is loaded with too much sample.
(c) The column is eluted first with 5% diethyl ether in hexanes, then the eluting solvent is immediately switched to 50% diethyl ether in hexanes.
(d) The column is allowed to go dry before it is completely developed.

Unlock Deck
Unlock for access to all 7 flashcards in this deck.
Unlock Deck
k this deck
5
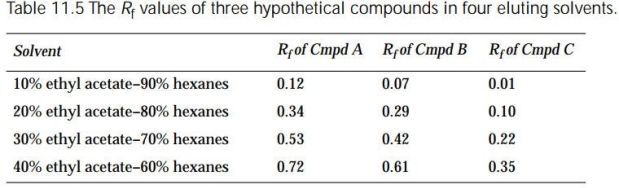
The Rf values determined on silica gel TLC plates for Compounds A, B, and C are tabulated in Table 11.5.
(a) In order to purify Compound A, which of the solvent systems should be used to develop the column in a flash chromatography procedure?
(b) Could 90 mg of a mixture of Compound A and Compound B be separated by macroscale flash column chromatography using 20% ethyl acetate-80% hexanes as the eluting sol- vent?
(c) Could 90 mg of a mixture of Compound A and Compound C be separated by macroscale flash column chromatography using 20% ethyl acetate-80% hexanes as the eluting sol- vent?
(a) In order to purify Compound A, which of the solvent systems should be used to develop the column in a flash chromatography procedure?
(b) Could 90 mg of a mixture of Compound A and Compound B be separated by macroscale flash column chromatography using 20% ethyl acetate-80% hexanes as the eluting sol- vent?
(c) Could 90 mg of a mixture of Compound A and Compound C be separated by macroscale flash column chromatography using 20% ethyl acetate-80% hexanes as the eluting sol- vent?

Unlock Deck
Unlock for access to all 7 flashcards in this deck.
Unlock Deck
k this deck
6
Of Compounds A, B, and C presented in Table 11.5, which is the most polar? The least polar?

Unlock Deck
Unlock for access to all 7 flashcards in this deck.
Unlock Deck
k this deck
7
Outline a procedure for separating Compound A from Compound C by flash chromatography using the data presented in Table 11.5. (Hint: First elute the less polar compound from the col- umn and then elute the more polar compound.)


Unlock Deck
Unlock for access to all 7 flashcards in this deck.
Unlock Deck
k this deck


